Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles for Improved Methane Storage Capacity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Hollow ZIF-8 Materials
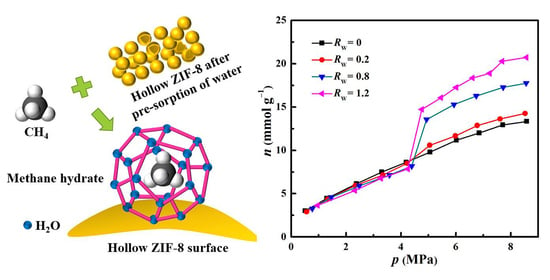
2.2. Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles
2.3. Formation Enthalpy of Methane Hydrate in Hollow ZIF-8 Nanoparticles
2.4. Stability of Hollow ZIF-8 Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Preparation of PS Microspheres
3.3. Preparation of Hollow ZIF-8 Nanoparticles
3.4. Characterizations
3.5. Methane Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, K.; Miller, J.B.; Huber, G.W.; Dumesic, J.A.; Maravelias, C.T. A General Framework for the Evaluation of Direct Nonoxidative Methane Conversion Strategies. Joule 2018, 2, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of Natural Gas Hydrates as an Energy Resource: Prospects and Challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane Hydrate Formation in Confined Nanospace Can Surpass Nature. Nat. Commun. 2015, 6, 6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, G.; Goh, M.N.; Arumuganainar, S.E.K.; Zhang, Y.; Linga, P. Ultra-Rapid Uptake and the Highly Stable Storage of Methane as Combustible Ice. Energy Environ. Sci. 2020, 13, 4946–4961. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Kumar, R.; Linga, P. An Innovative Approach to Enhance Methane Hydrate Formation Kinetics with Leucine for Energy Storage Application. Appl. Energy 2017, 188, 190–199. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental Principles and Applications of Natural Gas Hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Liu, B.; Liu, H.; Yang, Y.; Sun, C.; Chen, G. A Novel Method to Improve the Gas Storage Capacity of ZIF-8. J. Mater. Chem. 2012, 22, 12246–12252. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Mouchaham, G.; Daemen, L.; Cheng, Y.; Ramirez-Cuesta, A.; Aggarwal, H.; Missyul, A.; Eddaoudi, M.; Belmabkhout, Y.; Silvestre-Albero, J. Quest for an Optimal Methane Hydrate Formation in the Pores of Hydrolytically Stable Metal-organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13391–13397. [Google Scholar] [CrossRef]
- Casco, M.E.; Zhang, E.; Grätz, S.; Krause, S.; Bon, V.; Wallacher, D.; Grimm, N.; Többens, D.M.; Hauß, T.; Borchardt, L. Experimental Evidence of Confined Methane Hydrate in Hydrophilic and Hydrophobic Model Carbons. J. Phys. Chem. C 2019, 123, 24071–24079. [Google Scholar] [CrossRef]
- Borchardt, L.; Casco, M.E.; Silvestre-Albero, J. Methane Hydrate in Confined Spaces: An Alternative Storage System. ChemPhysChem 2018, 19, 1298–1314. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal-organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional Metal-organic Frameworks: From Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, D.F.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [Green Version]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Casco, M.E.; Rey, F.; Jordá, J.L.; Rudić, S.; Fauth, F.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. Paving the Way for Methane Hydrate Formation on Metal–Organic Frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhang, K.; Jiang, J. Formation of CH4 Hydrate in a Mesoporous Metal-organic Framework MIL-101: Mechanistic Insights from Microsecond Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2019, 10, 7002–7008. [Google Scholar] [CrossRef] [PubMed]
- Denning, S.; Majid, A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal-organic Framework HKUST-1 Promotes Methane Hydrate Formation for Improved Gas Storage Capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane Hydrate Growth Promoted by Microporous Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67 for Enhanced Methane Storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Lai, X.; Halpert, J.E.; Wang, D. Recent Advances in Micro-/Nano-Structured Hollow Spheres for Energy Applications: From Simple to Complex Systems. Energy Env. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Chen, M.; Ye, C.; Zhou, S.; Wu, L. Recent Advances in Applications and Performance of Inorganic Hollow Spheres in Devices. Adv. Mater. 2013, 25, 5343–5351. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Y.G.; Li, S.H.; Kong, L.Y.; Liu, H.O.; Li, Y.S.; Han, W.; Yeung, K.L.; Zhu, W.D.; Yang, W.S.; et al. New Membrane Architecture with High Performance: ZIF-8 Membrane Supported on Vertically Aligned ZnO Nanorods for Gas Permeation and Separation. Chem. Mater. 2014, 26, 1975–1981. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, W.; Oh, M. Advanced Fabrication of Metal-organic Frameworks: Template-Directed Formation of Polystyrene@ZIF-8 Core-shell and Hollow ZIF-8 Microspheres. Chem. Commun. 2012, 48, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.W. Imperfection Sensitivity of Externally Pressurized Spherical Shells. J. Appl. Mech. 1967, 34, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Shan, A.X.; Chen, Z.C.; Li, B.Q.; Chen, C.P.; Wang, R.M. Monodispersed, Ultrathin NiPt Hollow Nanospheres with Tunable Diameter and Composition via a Green Chemical Synthesis. J. Mater. Chem. A 2015, 3, 1031–1036. [Google Scholar] [CrossRef]
- Pan, Y.C.; Liu, Y.Y.; Zeng, G.F.; Zhao, L.; Lai, Z.P. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals in an Aqueous System. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, F.W.; Yang, Q.H.; Hu, Y.L.; Yan, H.; Chen, Y.Z.; Liu, H.R.; Zhang, G.Q.; Lu, J.L.; Jiang, H.L.; et al. Hollow Metal–Organic Framework Nanospheres via Emulsion-Based Interfacial Synthesis and Their Application in Size-Selective Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 18163–18171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Li, L.; Xiao, Z.X.; Liu, D.; Wang, S.; Zhang, J.J.; Hao, Y.T.; Zhang, W.Z. Hollow Sphere TiO2–ZrO2 Prepared by Self-Assembly with Polystyrene Colloidal Template for Both Photocatalytic Degradation and H2 Evolution from Water Splitting. ACS Sustain. Chem. Eng. A 2016, 4, 2037–2046. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Long, J.R.; Bae, T.-H. Carbon Dioxide Capture in Metal-organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Chen, S.; Fu, Y.; Zhang, Y.; Wang, D.; Pei, J.; Liu, D. Molecular Insights into Hybrid CH4 Physisorption-Hydrate Growth in Hydrophobic Metal-organic Framework ZIF-8: Implications for CH4 Storage. Chem. Eng. J. 2022, 430, 132901. [Google Scholar] [CrossRef]
- Im, S.H.; Lim, Y.T.; Suh, D.J.; Park, O.O. Three-Dimensional Self-Assembly of Colloids at a Water-Air Interface: A Novel Technique for the Fabrication of Photonic Bandgap Crystals. Adv. Mater. 2002, 14, 1367–1369. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Zhou, Y. Enhancement of the Methane Storage on Activated Carbon by Preadsorbed Water. AIChE J. 2002, 48, 2412–2416. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.; Sun, Y.; Li, J.; Zhou, Y. Methane Sorption in Ordered Mesoporous Silica SBA-15 in the Presence of Water. J. Phys. Chem. B 2005, 109, 22710–22714. [Google Scholar] [CrossRef] [PubMed]










| Sample | SBET (m2/g) | V (cm3/g) |
|---|---|---|
| PS@ZIF-8 | 518.8239 | 0.3187 |
| hollow ZIF-8 | 1260.9272 | 0.8250 |
| T (K) | p (MPa) | ϕ | lnƒ |
|---|---|---|---|
| 273 | 3.61 | 0.9303 | 1.2115 |
| 275 | 4.93 | 0.9007 | 1.4907 |
| 277 | 5.87 | 0.8803 | 1.6424 |
| 279 | 6.98 | 0.8583 | 1.7902 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Li, Y.; Cao, J. Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles for Improved Methane Storage Capacity. Catalysts 2022, 12, 485. https://doi.org/10.3390/catal12050485
Chen C, Li Y, Cao J. Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles for Improved Methane Storage Capacity. Catalysts. 2022; 12(5):485. https://doi.org/10.3390/catal12050485
Chicago/Turabian StyleChen, Chong, Yun Li, and Jilin Cao. 2022. "Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles for Improved Methane Storage Capacity" Catalysts 12, no. 5: 485. https://doi.org/10.3390/catal12050485