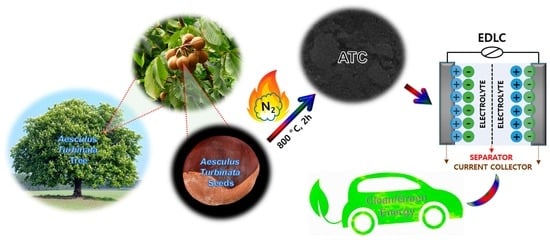
Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Preparation of ATC Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, H. Clean energy policies and green jobs: An evaluation of green jobs in US metropolitan areas. Energy Policy 2013, 56, 644–652. [Google Scholar] [CrossRef]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Harris, J.M.; Roach, B. Environmental and Natural Resource Economics: A Contemporary Approach; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M.; Ross, J. A nickel metal hydride battery for electric vehicles. Science 1993, 260, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Njoku, C.E.; Ike, I.S.; Adediran, A.A.; Nwaeju, C.C. The Place of Biomass-Based Electrode Materials in Next-Generation Energy Conversion and Storage. In Electrode Materials for Energy Storage and Conversion; CRC Press: Boca Raton, FL, USA, 2021; pp. 229–261. [Google Scholar]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Chandrasekaran, P.; Perumal, S.; Arunachalam, P.; Raja, P.B.; Sethuraman, M.G.; Lee, Y.R. Electrochemically exfoliated graphene sheets as electrode material for aqueous symmetric supercapacitors. Surf. Coat. Technol. 2021, 416, 127150. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Horn, M.; MacLeod, J.; Liu, M.; Webb, J.; Motta, N. Supercapacitors: A new source of power for electric cars? Econ. Anal. Policy 2019, 61, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, Y.; Beirne, S.; Razal, J.; Chen, J. Recent development of fabricating flexible micro-supercapacitors for wearable devices. Adv. Mater. Technol. 2018, 3, 1800028. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly stable transparent conductive silver grid/PEDOT: PSS electrodes for integrated bifunctional flexible electrochromic supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z. Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Vijayan, B.L.; Mohd Zain, N.K.; Misnon, I.I.; Reddy, M.V.; Adams, S.; Yang, C.-C.; Anilkumar, G.M.; Jose, R. Void space control in porous carbon for high-density supercapacitive charge storage. Energy Fuels 2020, 34, 5072–5083. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, T.; Xu, Y.; Graf, R.; Brunklaus, G.; Forster, M.; Chen, Y.; Scherf, U. Engineering the morphology of carbon materials: 2D porous carbon nanosheets for high-performance supercapacitors. ChemElectroChem 2016, 3, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and non-aqueous electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Babu, R.S.; Sundramoorthy, A.K.; Renita, A.A.; Lee, Y.R. Facile synthesis of nitrogen-doped porous carbon materials using waste biomass for energy storage applications. Chemosphere 2022, 289, 133225. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Xu, P.; Tong, J.; Zhang, L.; Yang, Y.; Chen, X.; Wang, J.; Zhang, S. Dung beetle forewing-derived nitrogen and oxygen self-doped porous carbon for high performance solid-state supercapacitors. J. Alloy Compd. 2022, 892, 162129. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Thirukumaran, P.; Vinodh, R.; Lee, Y.R. Green synthesis of nitrogen-doped carbon nanograss for supercapacitors. J. Taiwan Inst. Chem. Eng. 2019, 102, 475–486. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Lu, H.; Liu, S.; Zhang, Y.; Huang, Y.; Zhang, C.; Liu, T. Nitrogen-Doped Carbon Polyhedra Nanopapers: An Advanced Binder-Free Electrode for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 5240–5248. [Google Scholar] [CrossRef]
- Liu, X.; Mi, R.; Yuan, L.; Yang, F.; Fu, Z.; Wang, C.; Tang, Y. Nitrogen-Doped Multi-Scale Porous Carbon for High Voltage Aqueous Supercapacitors. Front. Chem. 2018, 6, 475. [Google Scholar] [CrossRef] [Green Version]
- Shaheen Shah, S.; Abu Nayem, S.M.; Sultana, N.; Saleh Ahammad, A.J.; Abdul Aziz, M. Preparation of Sulfur-doped Carbon for Supercapacitor Applications: A Review. ChemSusChem 2022, 15, e202101282. [Google Scholar] [CrossRef]
- Sato, I.; Suzuki, T.; Kobayashi, H.; Tsuda, S. Antioxidative and antigenotoxic effects of Japanese horse chestnut (Aesculus turbinata) seeds. J. Vet. Med. Sci. 2005, 67, 731–734. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Gizem Güneştekin, B.; Medetalibeyoglu, H.; Atar, N.; Lütfi Yola, M. Efficient Direct-Methanol Fuel Cell Based on Graphene Quantum Dots/Multi-walled Carbon Nanotubes Composite. Electroanalysis 2020, 32, 1977–1982. [Google Scholar] [CrossRef]
- Titus, E.; Ali, N.; Cabral, G.; Gracio, J.; Babu, P.R.; Jackson, M. Chemically functionalized carbon nanotubes and their characterization using thermogravimetric analysis, fourier transform infrared, and raman spectroscopy. J. Mater. Eng. Perform. 2006, 15, 182–186. [Google Scholar] [CrossRef]
- Tong, X.; Qin, Y.; Guo, X.; Moutanabbir, O.; Ao, X.; Pippel, E.; Zhang, L.; Knez, M. Enhanced catalytic activity for methanol electro-oxidation of uniformly dispersed nickel oxide nanoparticles—Carbon nanotube hybrid materials. Small 2012, 8, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Lee, Y.R. Indian gooseberry-derived tunable fluorescent carbon dots as a promise for in vitro/in vivo multicolor bioimaging and fluorescent ink. ACS Omega 2018, 3, 17590–17601. [Google Scholar] [CrossRef]
- Dam, D.T.; Wang, X.; Lee, J.-M. Graphene/NiO nanowires: Controllable one-pot synthesis and enhanced pseudocapacitive behavior. ACS Appl. Mater. Interfaces 2014, 6, 8246–8256. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, P.; Atchudan, R.; Perumal, S.; Salama, E.-S.; Lee, Y.R.; Jeon, B.-H. Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 2022, 286, 131764. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Lee, Y.R. Betel-derived nitrogen-doped multicolor carbon dots for environmental and biological applications. J. Mol. Liq. 2019, 296, 111817. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, L.; Fang, C.; Li, T.; Chen, J.; Yang, M.; Zhang, Q. Synthesis of porous carbon materials derived from laminaria japonica via simple carbonization and activation for supercapacitors. J. Mater. Res. Technol. 2020, 9, 3261–3271. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Liu, G. Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials. Appl. Sci. 2020, 10, 422. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Lv, C.; Watanabe, A. High-performance all-solid-state flexible carbon/TiO2 micro-supercapacitors with photo-rechargeable capability. RSC Adv. 2017, 7, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Zhou, L.; Mak, C.L.; Huang, H.; Tang, W.M.; Chan, H.L.W. Improved performance of asymmetric fiber-based micro-supercapacitors using carbon nanoparticles for flexible energy storage. J. Mater. Chem. A 2015, 3, 15633–15641. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, R.K.; Shinde, N.M.; Yun, J.M.; Mane, R.S.; Kim, K.H. Synthesis of Bi2O3-MnO2 nanocomposite electrode for wide-potential window high performance supercapacitor. Energies 2019, 12, 3320. [Google Scholar] [CrossRef] [Green Version]
- Sahu, V.; Shekhar, S.; Ahuja, P.; Gupta, G.; Singh, S.K.; Sharma, R.K.; Singh, G. Synthesis of hydrophilic carbon black; role of hydrophilicity in maintaining the hydration level and protonic conduction. RSC Adv. 2013, 3, 3917–3924. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kaushik, S.; Gairola, P.; Gairola, S.P. Polyaniline enfolded hybrid carbon array substrate electrode for high performance supercapacitors. J. Polym. Eng. 2019, 39, 228–238. [Google Scholar] [CrossRef]
- Raji, A.; Thomas Nesakumar, J.I.E.; Mani, S.; Perumal, S.; Rajangam, V.; Thirunavukkarasu, S.; Lee, Y.R. Biowaste-originated heteroatom-doped porous carbonaceous material for electrochemical energy storage application. J. Ind. Eng. Chem. 2021, 98, 308–317. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Abd Aziz, R.; Vidyadharan, B.; Jose, R. Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wan, S.; Jiang, X.; Wang, Z.; Gao, S. Peanut-shell-like porous carbon from nitrogen-containing poly-N-phenylethanolamine for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22238–22245. [Google Scholar] [CrossRef]
- Mo, R.-J.; Zhao, Y.; Wu, M.; Xiao, H.-M.; Kuga, S.; Huang, Y.; Li, J.-P.; Fu, S.-Y. Activated carbon from nitrogen rich watermelon rind for high-performance supercapacitors. RSC Adv. 2016, 6, 59333–59342. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Chen, Y.; Guo, H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Z.; Du, L.; Fan, X.; Shen, Z.; Ren, X.; Zhao, Y.; Wei, C.; Wei, S. Designing synthesis of porous biomass carbon from wheat straw and the functionalizing application in flexible, all-solid-state supercapacitors. J. Alloy Compd. 2019, 797, 1031–1040. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, L.; Liu, Q.; Sun, H.; Wang, Q.; Li, W.; Li, Z.; Wang, B. Ultra-high specific surface area porous carbon derived from chestnut for high-performance supercapacitor. Biomass Bioenergy 2021, 153, 106227. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Kaur, A.; Maheshwari, P.H.; Sundriyal, S. Surface and Diffusion Charge Contribution Studies of Human Hair-Derived Heteroatom-Doped Porous Carbon Electrodes for Supercapacitors. Energy Fuels 2021, 36, 626–637. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.; Su, S.; Cui, Y.; Shi, W.; Zhu, X. Porous active carbon derived from lotus stalk as electrode material for high-performance supercapacitors. J. Wood Chem. Technol. 2021, 41, 46–57. [Google Scholar] [CrossRef]
- Pang, L.; Zou, B.; Zou, Y.; Han, X.; Cao, L.; Wang, W.; Guo, Y. A new route for the fabrication of corn starch-based porous carbon as electrochemical supercapacitor electrode material. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 26–33. [Google Scholar] [CrossRef]








| Precursor | Synthesis Method | Electrolyte Solution | Cs (F g−1)/ CD (A g−1) | Cycle/Retention (%) | Reference |
|---|---|---|---|---|---|
| Banana peel | carbonization | 1 M H2SO4 | 137/0.5 | 10,000/94 | [50] |
| Palm kernel shell | carbonization | 1 M KOH | 210/0.5 | 1000/97 | [51] |
| Peanut shell | carbonization | 1 M H2SO4 | 340/1.0 | 10,000/95.3 | [52] |
| Watermelon rind | carbonization | 6 M KOH | 333.4/1.0 | 10,000/96.8 | [53] |
| Bamboo | carbonization | 3 M KOH | 293/0.5 | 10,000/91.8 | [54] |
| Wheat straw | citric-acid-crosslinking | (PVA)/KOH | 200/10 | 5000/97.6 | [55] |
| Chestnut-pulp | carbonization | 6 M KOH | 373/0.5 | 10,000/99.7 | [56] |
| Human hair | carbonization | 1 M H2SO4 | 274.5/1.0 | 10,000/87 | [57] |
| Lotus stalk | carbonization | 6 M KOH | 248.5/1.0 | 5000/90.6 | [58] |
| Lotus fruit | carbonization | 1 M H2SO4 | 160/0.5 | 10,000/95 | [24] |
| Corn starch | carbonization | 6 M KOH | 144/0.6 | 3000/99 | [59] |
| A. turbinata seed | carbonization | 1 M H2SO4 | 142/0.5 | 10,000/95 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Chandra Kishore, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting. Catalysts 2022, 12, 436. https://doi.org/10.3390/catal12040436
Perumal S, Chandra Kishore S, Atchudan R, Sundramoorthy AK, Alagan M, Lee YR. Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting. Catalysts. 2022; 12(4):436. https://doi.org/10.3390/catal12040436
Chicago/Turabian StylePerumal, Suguna, Somasundaram Chandra Kishore, Raji Atchudan, Ashok K. Sundramoorthy, Muthulakshmi Alagan, and Yong Rok Lee. 2022. "Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting" Catalysts 12, no. 4: 436. https://doi.org/10.3390/catal12040436