Hybrid Catalysts from Copper Biosorbing Bacterial Strains and Their Recycling for Catalytic Application in the Asymmetric Addition Reaction of B2(pin)2 on α,β-Unsaturated Chalcones
Abstract
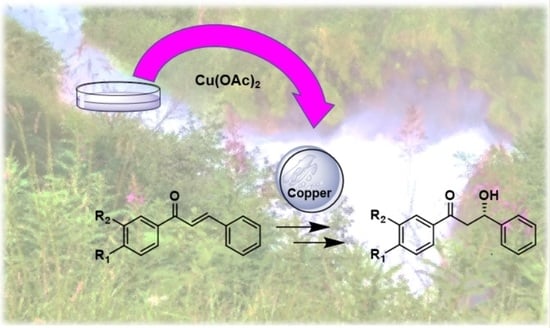
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, E.; Colica, G.; Viti, C.; Tamagnini, P.; De Philippis, R. Selectivity in the heavy metal removal by exopolysaccharide-producing cyanobacteria. J. Appl. Microbiol. 2008, 105, 88–94. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shouny, W.A.; Osman, M.E.; El-Gammal, E. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Reis, M.A.M.; Freitas, F. Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol. Appl. Sci. 2020, 10, 6708. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Metal(loid) Bioremediation: Strategies Employed by Microbial Polymers. Sustainability 2018, 10, 3028. [Google Scholar] [CrossRef]
- Fang, L.; Yang, S.; Huang, Q.; Xue, A.; Cai, P. Biosorption mechanisms of Cu(II) by extracellular polymeric substances from Bacillus subtilis. Chem. Geol. 2014, 386, 143–151. [Google Scholar] [CrossRef]
- Chug, R.; Mathur, S.; Kothari, S.L.; Gour, V.S. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem. Biophys. Rep. 2021, 26, 100972. [Google Scholar] [CrossRef]
- Casentini, B.; Gallo, M.; Baldi, F. Arsenate and arsenite removal from contaminated water by iron oxides nanoparticles formed inside a bacterial exopolysaccharide. J. Environ. Chem. Eng. 2019, 7, 102908. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Shiri, P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, 5600. [Google Scholar] [CrossRef]
- Zhang, Z.; Górski, B.; Leonori, D. Merging Halogen-Atom Transfer (XAT) and Copper Catalysis for the Modular Suzuki–Miyaura-Type Cross-Coupling of Alkyl Iodides and Organoborons. J. Am. Chem. Soc. 2022, 144, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xue, Y.; Hu, W.; Shi, L.; Zhu, X.; Hao, X.-Q.; Song, M.-P. Cu(II)-Catalyzed N-Directed Distal C(sp3)–H Heteroarylation of Aliphatic N-Fluorosulfonamides. Org. Lett. 2022, 24, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef]
- Facchetti, G.; Bucci, R.; Fusè, M.; Rimoldi, I. Asymmetric Hydrogenation vs. Transfer Hydrogenation in the Reduction of Cyclic Imines. ChemistrySelect 2018, 3, 8797–8800. [Google Scholar] [CrossRef]
- Gandolfi, R.; Facchetti, G.; Christodoulou, M.S.; Fusè, M.; Meneghetti, F.; Rimoldi, I. Cascade Reaction by Chemo- and Biocatalytic Approaches to Obtain Chiral Hydroxy Ketones and anti 1,3-Diols. ChemistryOpen 2018, 7, 393–400. [Google Scholar] [CrossRef]
- Facchetti, G.; Fusè, M.; Pecoraro, T.; Nava, D.; Rimoldi, I. New sp3 diphosphine-based rhodium catalysts for the asymmetric conjugate addition of aryl boronic acids to 3-azaarylpropenones. New J. Chem. 2021, 45, 18769–18775. [Google Scholar] [CrossRef]
- Iacovino, L.G.; Pinzi, L.; Facchetti, G.; Bortolini, B.; Christodoulou, M.S.; Binda, C.; Rastelli, G.; Rimoldi, I.; Passarella, D.; Di Paolo, M.L.; et al. Promising Non-cytotoxic Monosubstituted Chalcones to Target Monoamine Oxidase-B. ACS Med. Chem. Lett. 2021, 12, 1151–1158. [Google Scholar] [CrossRef]
- Rimoldi, I.; Bucci, R.; Feni, L.; Santagostini, L.; Facchetti, G.; Pellegrino, S. Exploring the copper binding ability of Mets7 hCtr-1 protein domain and His7 derivative: An insight in Michael addition catalysis. J. Pept. Sci. 2021, 27, e3289. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, G.; Temml, V.; Peralta, A.C.; Gruet, O.; Richomme, P.; Séraphin, D.; Viault, G.; Kraus, L.; Huber-Cantonati, P.; Schopfhauser, E.; et al. Analogues of Natural Chalcones as Efficient Inhibitors of AKR1C3. Metabolites 2022, 12, 99. [Google Scholar] [CrossRef]
- Wilhelm, A.; Bonnet, S.L.; Twigge, L.; Rarova, L.; Stenclova, T.; Visser, H.G.; Schutte-Smith, M. Synthesis, characterization and cytotoxic evaluation of chalcone derivatives. J. Mol. Struct. 2022, 1251, 132001. [Google Scholar] [CrossRef]
- Ammaji, S.; Masthanamma, S.; Bhandare, R.R.; Annadurai, S.; Shaik, A.B. Antitubercular and antioxidant activities of hydroxy and chloro substituted chalcone analogues: Synthesis, biological and computational studies. Arab. J. Chem. 2021, 15, 103581. [Google Scholar] [CrossRef]
- Yadav, M.; Lal, K.; Kumar, A.; Kumar, A.; Kumar, D. Indole-chalcone linked 1,2,3-triazole hybrids: Facile synthesis, antimicrobial evaluation and docking studies as potential antimicrobial agents. J. Mol. Struct. 2022, 1261, 132867. [Google Scholar] [CrossRef]
- Rammohan, A.; Bhaskar, B.V.; Venkateswarlu, N.; Gu, W.; Zyryanov, G.V. Design, synthesis, docking and biological evaluation of chalcones as promising antidiabetic agents. Bioorg. Chem. 2020, 95, 103527. [Google Scholar] [CrossRef]
- Mughal, E.U.; Obaid, R.J.; Sadiq, A.; Alsharif, M.A.; Naeem, N.; Kausar, S.; Altaf, A.A.; Jassas, R.S.; Ahmed, S.; Alsantali, R.I.; et al. Chalcone- and flavone-based novel terpyridine metal complexes: Synthesis, electrochemical, photophysical, photovoltaic and computational studies. Dye. Pigment. 2022, 201, 110248. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone De-rivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [Green Version]
- Cavalca, L.; Zecchin, S.; Zaccheo, P.; Abbas, B.; Rotiroti, M.; Bonomi, T.; Muyzer, G. Exploring Biodiversity and Arsenic Metabolism of Microbiota Inhabiting Arsenic-Rich Groundwaters in Northern Italy. Front. Microbiol. 2019, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, R.; Okeke, B.C.; Pieniz, S.; Camargo, F. Characterization of Copper-Resistant Rhizosphere Bacteria from Avena sativa and Plantago lanceolata for Copper Bioreduction and Biosorption. Biol. Trace Elem. Res. 2011, 146, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; Naccari, C.; Nostro, A.; Pizzimenti, A.; Trombetta, D.; Pizzimenti, F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ. Sci. Pollut. Res. 2012, 19, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Marrero, J.; Cabrera, G.; Coto, O.; Gómez, J.M. Biosorption of nickel, cobalt, zinc and copper ions by Serratia mar-cescens strain 16 in mono and multimetallic systems. Biodegradation 2021, 33, 33–43. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Marques, F.; Matos, A.P.; Dos Santos, M.M.C.; Azevedo, C.G.; Capelo, J.-L.; Santos, H.M.; Gama, S.; Pinheiro, T.; et al. Copper Complexes with 1,10-Phenanthroline Derivatives: Underlying Factors Affecting Their Cytotoxicity. Inorg. Chem. 2020, 59, 9116–9134. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Facchetti, G.; Contini, A.; Gelmi, M.L.; Erba, E.; Gandolfi, R.; Rimoldi, I. Ctr-1 Mets7 motif inspiring new peptide ligands for Cu(i)-catalyzed asymmetric Henry reactions under green conditions. RSC Adv. 2016, 6, 71529–71533. [Google Scholar] [CrossRef] [Green Version]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S. A Cu(ii)-based strategy for catalytic enantioselective β-borylation of α,β-unsaturated acceptors. Chem. Commun. 2015, 51, 11685–11688. [Google Scholar] [CrossRef]
- Kitanosono, T.; Xu, P.; Isshiki, S.; Zhu, L.; Kobayashi, S. Cu(ii)-Catalyzed asymmetric boron conjugate addition to α,β-unsaturated imines in water. Chem. Commun. 2014, 50, 9336–9339. [Google Scholar] [CrossRef]
- Quaglio, D.; Zhdanovskaya, N.; Tobajas, G.; Cuartas, V.; Balducci, S.; Christodoulou, M.S.; Fabrizi, G.; Gargantilla, M.; Priego, E.-M.; Pestaña Álvaro, C.; et al. Chalcones and Chalcone-mimetic Derivatives as Notch Inhibitors in a Model of T-cell Acute Lymphoblastic Leukemia. ACS Med. Chem. Lett. 2019, 10, 639–643. [Google Scholar] [CrossRef]
- Edwards, M.L.; Stemerick, D.M.; Sunkara, P.S. Chalcones: A new class of antimitotic agents. J. Med. Chem. 1990, 33, 1948–1954. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Lekkala, R.; Alsulami, H.; Rakesh, K. Chalcone hybrids as privileged scaffolds in antimalarial drug discovery: A key review. Eur. J. Med. Chem. 2020, 193, 112215. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Facchetti, G.; Esposito, S.; Maddalon, A.; Rimoldi, I.; Christodoulou, M.S. Antiproliferative effects of chalcones on T cell acute lymphoblastic leukemia-derived cells: Role of PKCβ. Arch. Pharm. 2020, 353, e2000062. [Google Scholar] [CrossRef] [PubMed]
- Illa, O.; Porcar-Tost, O.; Robledillo, C.; Elvira, C.; Nolis, P.; Reiser, O.; Branchadell, V.; Ortuño, R.M. Stereoselectivity of Proline/Cyclobutane Amino Acid-Containing Peptide Organocatalysts for Asymmetric Aldol Additions: A Rationale. J. Org. Chem. 2018, 83, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Heidlindemann, M.; Berkessel, A.; Gröger, H. Side-Product Catalysis”: Substrate Autoxidation as an Overlooked Side Reaction Generating a Co-Catalyst for Enhancing Asymmetric Aldol Reactions. ChemCatChem 2017, 9, 1383–1388. [Google Scholar] [CrossRef]






| Bacteria | Atmosphere | Molar Conversion (%) | e.e. (%) |
|---|---|---|---|
| 13a | inert | 47 | 12 |
| 13a | air | 19 | 14 |
| SC5II | inert | 72 | 15 |
| SC5II | air | 13 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfi, R.; Facchetti, G.; Cavalca, L.; Mazzini, S.; Colombo, M.; Coffetti, G.; Borgonovo, G.; Scaglioni, L.; Zecchin, S.; Rimoldi, I. Hybrid Catalysts from Copper Biosorbing Bacterial Strains and Their Recycling for Catalytic Application in the Asymmetric Addition Reaction of B2(pin)2 on α,β-Unsaturated Chalcones. Catalysts 2022, 12, 433. https://doi.org/10.3390/catal12040433
Gandolfi R, Facchetti G, Cavalca L, Mazzini S, Colombo M, Coffetti G, Borgonovo G, Scaglioni L, Zecchin S, Rimoldi I. Hybrid Catalysts from Copper Biosorbing Bacterial Strains and Their Recycling for Catalytic Application in the Asymmetric Addition Reaction of B2(pin)2 on α,β-Unsaturated Chalcones. Catalysts. 2022; 12(4):433. https://doi.org/10.3390/catal12040433
Chicago/Turabian StyleGandolfi, Raffaella, Giorgio Facchetti, Lucia Cavalca, Stefania Mazzini, Milena Colombo, Giulia Coffetti, Gigliola Borgonovo, Leonardo Scaglioni, Sarah Zecchin, and Isabella Rimoldi. 2022. "Hybrid Catalysts from Copper Biosorbing Bacterial Strains and Their Recycling for Catalytic Application in the Asymmetric Addition Reaction of B2(pin)2 on α,β-Unsaturated Chalcones" Catalysts 12, no. 4: 433. https://doi.org/10.3390/catal12040433