Kinetic Study and Modeling of the Degradation of Aqueous Ammonium/Ammonia Solutions by Heterogeneous Photocatalysis with TiO2 in a UV-C Pilot Photoreactor
Abstract
:1. Introduction
2. Results
2.1. Kinetic Study of Ammonia Removal
2.2. Influence of Different Factors on the Heterogenous Photocatalytic Process of NH4+/NH3 Removal
2.3. Influence of Stripping, Photocatalysis and Photolysis on the Kinetics of Ammonia Removal
2.4. Initial Ammonia Removal Rates at pH = 11.0 and 25 W UVC Ultraviolet Lamp Irradiation Power. Models Discrimination
2.5. Study of the Photocatalytic Fiber Stability after Ammonium/Ammonia Removal Process
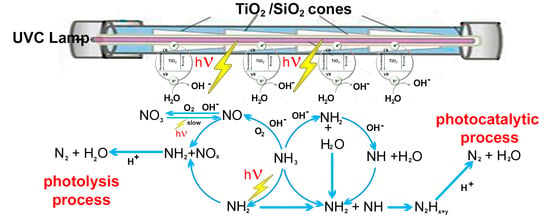
2.6. A Two Parallel Reaction Mechanism for Ammonium Degradation by Photolysis and Photocatalysis
3. Materials and Methods
3.1. TiO2/SiO2 Fixed Bed Photoreactor with Total Recirculation
3.2. Experimental Conditions
3.3. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency (EEA). NEC Directive Reporting Status; EEA: Copenhagen, Denmark, 2019. [Google Scholar] [CrossRef]
- Low, G.K.C.; McEvoy, S.R.; Matthews, R.W. Formation of nitrate and ammonium ions in titanium dioxide mediated photocatalytic degradation of organic compounds containing nitrogen atoms. Environ. Sci. Technol. 1991, 25, 460–467. [Google Scholar] [CrossRef]
- Manahan, S.E. Environmental Chemistry, 1st ed.; Lewis Publishers: Chelsea, UK, 1991; pp. 128–132. [Google Scholar]
- Randall, D.; Tsui, T. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 2nd ed.; WHO: Geneva, Switzerland, 1996; Volume 2. [Google Scholar]
- Agarwal, D.C. Stress corrosion in copper–nickel alloys: Influence of ammonia. Br. Corros. J. 2002, 37, 267–275. [Google Scholar] [CrossRef]
- Huang, H.; Song, Q.; Wang, W.; Wu, S.; Dai, J. Treatment of anaerobic digester effluents of nylon wastewater through chemical precipitation and a sequencing batch reactor process. J. Environ. Manag. 2012, 101, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Lin, H.; Dong, Y.; Cheng, H.; Wang, H.; Cao, L. Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. J. Hazard. Mater. 2012, 229-230, 292–297. [Google Scholar] [CrossRef]
- Değermenci, N.; Ata, O.N.; Yildiz, E. Ammonia removal by air stripping in a semi-batch jet loop reactor. J. Ind. Eng. Chem. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Stoquart, C.; Servais, P.; Bérubé, P.R.; Barbeau, B. Hybrid Membrane Processes using activated carbon treatment for drinking water: A review. J. Membr. Sci. 2012, 411-412, 1–12. [Google Scholar] [CrossRef]
- Chauhan, R.; Srivastava, V.C. Electrochemical denitrification of highly contaminated actual nitrate wastewater by Ti/RuO2 anode and iron cathode. Chem. Eng. J. 2019, 386, 122065. [Google Scholar] [CrossRef]
- Mulder, A.; Van De Graaf, A.; Robertson, L.; Kuenen, J. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L.; Winfrey, B.K.; Wang, J.; Gong, J. Treatment of rich ammonia nitrogen wastewater with polyvinyl alcohol immobilized nitrifier biofortified constructed wetlands. Ecol. Eng. 2016, 94, 7–11. [Google Scholar] [CrossRef]
- Zhang, G.; Ruan, J.; Du, T. Recent Advances on Photocatalytic and Electrochemical Oxidation for Ammonia Treatment from Water/Wastewater. ACS EST Eng. 2020, 1, 310–325. [Google Scholar] [CrossRef]
- Ahn, Y.-H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.; Khadivi, M. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2013, 20, 278–283. [Google Scholar] [CrossRef]
- Bahadori, E.; Conte, F.; Tripodi, A.; Ramis, G.; Rossetti, I. Photocatalytic Selective Oxidation of Ammonia in a Semi-Batch Reactor: Unravelling the Effect of Reaction Conditions and Metal Co-Catalysts. Catalysts 2021, 11, 209. [Google Scholar] [CrossRef]
- Zendehzaban, M.; Sharifnia, S.; Hosseini, S.N. Photocatalytic degradation of ammonia by light expanded clay aggregate (LECA)-coating of TiO2 nanoparticles. Korean J. Chem. Eng. 2013, 30, 574–579. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.; Zhang, G.; Li, J.; Song, W.; Xu, Z. Improved photocatalytic conversion of high−concentration ammonia in water by low−cost Cu/TiO2 and its mechanism study. Chemosphere 2021, 274, 129689. [Google Scholar] [CrossRef] [PubMed]
- Mozzanega, H.; Herrmann, J.M.; Pichat, P. Ammonia oxidation over UV-irradiated titanium dioxide at room temperature. J. Phys. Chem. 1979, 83, 2251–2255. [Google Scholar] [CrossRef]
- Dijkstra, M.; Michorius, A.; Buwalda, H.; Panneman, H.; Winkelman, J.; Beenackers, A. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamaoka, H.; Harada, Y.; Fujii, T.; Nagasawa, T. A general process for in situ formation of functional surface layers on ceramics. Nature 2002, 416, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Chen, Z.-Y.; Bao, S.-J.; Wang, M.-Q.; Song, C.-L.; Pu, S.; Long, D. Ultrafine TiO2 encapsulated in nitrogen-doped porous carbon framework for photocatalytic degradation of ammonia gas. Chem. Eng. J. 2018, 331, 383–388. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, H.; Wang, A.; Si, Y. Preparation of hollow B–SiO2@TiO2 composites and their photocatalytic performances for degradation of ammonia-nitrogen and green algae in aqueous solution. Chin. J. Chem. Eng. 2019, 27, 2535–2543. [Google Scholar] [CrossRef]
- Peng, X.; Wang, M.; Hu, F.; Qiu, F.; Dai, H.; Cao, Z. Facile fabrication of hollow biochar carbon-doped TiO2/CuO composites for the photocatalytic degradation of ammonia nitrogen from aqueous solution. J. Alloy. Compd. 2018, 770, 1055–1063. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Chen, P.; Zhang, D.; Yang, S.; Liu, Z. Microstructure and photocatalytic properties of Ag/Ce4+/La3+ co-modified TiO2/Basalt fiber for ammonia–nitrogen removal from synthetic wastewater. J. Sol-Gel Sci. Technol. 2016, 82, 289–298. [Google Scholar] [CrossRef]
- Bravo, A.; Garcia, J.; Domenech, X.; Peral, J. Some Aspects of the Photocatalytic Oxidation of ammonium ion by Titanium Dioxide. J. Chem. Res. 1993, 1, 376–377. [Google Scholar]
- Bonsen, E.-M.; Schroeter, S.; Jacobs, H.; Broekaert, J. Photocatalytic degradation of ammonia with t102 as photocatalyst in the laboratory and under the use of solar radiation. Chemosphere 1997, 35, 1431–1445. [Google Scholar] [CrossRef]
- Zhu, X.; Castleberry, S.R.; Nanny, M.A.; Butler, E.C. Effects of pH and Catalyst Concentration on Photocatalytic Oxidation of Aqueous Ammonia and Nitrite in Titanium Dioxide Suspensions. Environ. Sci. Technol. 2005, 39, 3784–3791. [Google Scholar] [CrossRef]
- Sun, D.; Sun, W.; Yang, W.; Li, Q.; Shang, J.K. Efficient photocatalytic removal of aqueous NH4+–NH3 by palladium-modified nitrogen-doped titanium oxide nanoparticles under visible light illumination, even in weak alkaline solutions. Chem. Eng. J. 2015, 264, 728–734. [Google Scholar] [CrossRef]
- Altomare, M.; Dozzi, M.V.; Chiarello, G.L.; Di Paola, A.; Palmisano, L.; Selli, E. High activity of brookite TiO2 nanoparticles in the photocatalytic abatement of ammonia in water. Catal. Today 2015, 252, 184–189. [Google Scholar] [CrossRef]
- Shibuya, S.; Sekine, Y.; Mikami, I. Influence of pH and pH adjustment conditions on photocatalytic oxidation of aqueous ammonia under airflow over Pt-loaded TiO2. Appl. Catal. A Gen. 2015, 496, 73–78. [Google Scholar] [CrossRef]
- Vohra, M.; Selimuzzaman, S.; Al-Suwaiyan, M. NH 4+-NH 3 removal from simulated wastewater using UV-TiO2 photocatalysis: Effect of co-pollutants and pH. Environ. Technol. 2010, 31, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.E.; Alfano, O.M. Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal. Today 2000, 58, 167–197. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Chiarello, G.L.; Costa, A.; Guarino, M.; Selli, E. Photocatalytic abatement of ammonia in nitrogen-containing effluents. Chem. Eng. J. 2012, 191, 394–401. [Google Scholar] [CrossRef]
- Serewicz, A.; Noyes Aibert, W., Jr. The Photolysis of Ammonia in the Presence of Nitric Oxide. J. Phys. Chem. 1959, 63, 843–845. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Dong, W.; Liu, Y.; Hou, H. Removal of Ammonia by OH Radical in Aqueous Phase. Environ. Sci. Technol. 2008, 42, 8070–8075. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Chen, B.; Wang, L.; Zhu, R. Effects of pH and H2O2 on ammonia, nitrite and nitrate transformations during UV254nm irradiation: Implications to nitrogen removal and analysis. Chemosphere 2017, 184, 1003–1011. [Google Scholar] [CrossRef]
- Wang, S.; Ye, Z.; Taghipour, F. UV photoelectrochemical process for the synergistic degradation of total ammonia nitrogen (TAN). J. Clean. Prod. 2020, 289, 125645. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. Interfacial Electrochem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Murgia, S.M.; Poletti, A.; Selvaggi, R. Photocatalytic Degradation of High Ammonia Concentration Water Solutions by TiO2. Ann. Chim. 2005, 95, 335–343. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Choi, W. Selective Photocatalytic Oxidation of NH3 to N2 on Platinized TiO2 in Water. Environ. Sci. Technol. 2002, 36, 5462–5468. [Google Scholar] [CrossRef]
- Núñez-Núñez, C.M.; Chairez-Hernández, I.; García-Roig, M.; García-Prieto, J.C.; Melgoza-Alemán, R.M.; Proal-Nájera, J.B. UV-C/H2O2 heterogeneous photocatalytic inactivation of coliforms in municipal wastewater in a TiO2/SiO2 fixed bed reactor: A kinetic and statistical approach. React. Kinet. Mech. Catal. 2018, 125, 1159–1177. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Biyoghe Bi Ndong, L.; Ibondou, M.P.; Gu, X.; Lu, S.; Qiu, Z.; Sui, Q.; Mbadinga, S.M. Enhanced Photocatalytic Activity of TiO2 Nanosheets by Doping with Cu for Chlorinated Solvent Pollutants Degradation. Ind. Eng. Chem. Res. 2014, 53, 1368–1376. [Google Scholar] [CrossRef]
- Gong, X.; Wang, H.; Yang, C.; Li, Q.; Chen, X.; Hu, J. Photocatalytic degradation of high ammonia concentration wastewater by TiO2. Futur. Cities Environ. 2015, 1, 12. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Mu, W.; Pichat, P. Heterogeneous catalysis and fine chemicals II; Guisnet, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 405–420. [Google Scholar]
- Szabó-Bárdos, E.; Czili, H.; Horváth, A. Photocatalytic oxidation of oxalic acid enhanced by silver deposition on a TiO2 surface. J. Photochem. Photobiol. A Chem. 2003, 154, 195–201. [Google Scholar] [CrossRef]
- Zhu, X.; Nanny, M.A.; Butler, E.C. Effect of inorganic anions on the titanium dioxide-based photocatalytic oxidation of aqueous ammonia and nitrite. J. Photochem. Photobiol. A Chem. 2007, 185, 289–294. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Doudrick, K.; Yang, T.; Hristovski, K.; Westerhoff, P. Photocatalytic nitrate reduction in water: Managing the hole scavenger and reaction by-product selectivity. Appl. Catal. B Environ. 2013, 136–137, 40–47. [Google Scholar] [CrossRef]
- Krasae, N.; Wantala, K. Enhanced nitrogen selectivity for nitrate reduction on Cu–nZVI by TiO2 photocatalysts under UV irradiation. Appl. Surf. Sci. 2016, 380, 309–317. [Google Scholar] [CrossRef]
- García-Prieto, J.C.; González-Burciaga, L.A.; Proal-Nájera, J.B.; García-Roig, M. Study of Influence Factors in the Evaluation of the Performance of a Photocatalytic Fibre Reactor (TiO2/SiO2) for the Removal of Organic Pollutants from Water. Catalysts 2022, 12, 122. [Google Scholar] [CrossRef]
- Cho, S.; Kambey, C.; Nguyen, V.K. Performance of Anammox Processes for Wastewater Treatment: A Critical Review on Effects of Operational Conditions and Environmental Stresses. Water 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.; Al-Sayyed, G.; Srijaranai, S. Adsorption of Model Pollutants on TiO2 Particles in Relation to Photoremediation of Contaminated Water; Helz, G., Zepp, R., Crosby, D., Eds.; Aquatic and Surface Chemistry: Boca Raton, FL, USA, 1994; pp. 317–348. [Google Scholar]
- Ishikawa, T. Advances in inorganic fibres. In Polymeric and Inorganic Fibres; Springer: Berlin, Heidelberg, 2005; Volume 178, pp. 109–144. [Google Scholar] [CrossRef]
- Cachaza Silverio, E.; Diez Mateos, A.; García-Prieto, J.C. La fotocatálisis como tecnología para la desinfección del agua sin aditivos químicos. Ensayos del reactor fotocatalítico UBE en la inactivación de microorganismos. Tecnología del Agua. 2005, 261, 81–85. [Google Scholar]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 385–427. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, A.J.; Zhou, J. Research of Treating High Ammonia-N Wastewater by CASS Process. Adv. Mater. Res. 2011, 250–253, 3844–3847. [Google Scholar] [CrossRef]
- Bardsley, W.G. Simfit Statistical package. v. 7.3.1 Academic 64-bit University of Manchester UK. Available online: http://www.simfit.man.ac.uk (accessed on 14 December 2021).















| Initial Concentration (mM) | 1.57 | 2.67 | 6.41 | 13.82 |
| Surface coverage Θ | 0.008 | 0.013 | 0.032 | 0.07 |
| k1 (h−1) | k2 (h−1) | k3 (h−1) | |
|---|---|---|---|
| Photocatalysis 15 W pH = 7,0 | (1.1 ± 0.2) 10−3 | (1.5 ± 0.2) 10−2 | 0.016 |
| Photocatalysis 15 W pH = 9.0 | (8.2 ± 0.6) 10−6 | (2.1 ± 0.2) 10−2 | 0.021 |
| Photocatalysis 15 W pH = 11.0 | (2.2 ± 0.1) 10−5 | (1.03 ± 0.09) 10−1 | 0.103 |
| Photocatalysis 25 W pH = 7.0 | (1.5 ± 0.2) 10−3 | (1.9 ± 0.5) 10−2 | 0.021 |
| Photocatalysis 25 W pH = 9.0 | (8.7 ± 0.6) 10−7 | (5.1 ± 0.3) 10−2 | 0.051 |
| Photocatalysis 25 W pH = 11.0 | (3.3 ± 0.2) 10−5 | (1.54 ± 0.07) 10−1 | 0.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Prieto, J.C.; González-Burciaga, L.A.; Proal-Nájera, J.B.; García-Roig, M. Kinetic Study and Modeling of the Degradation of Aqueous Ammonium/Ammonia Solutions by Heterogeneous Photocatalysis with TiO2 in a UV-C Pilot Photoreactor. Catalysts 2022, 12, 352. https://doi.org/10.3390/catal12030352
García-Prieto JC, González-Burciaga LA, Proal-Nájera JB, García-Roig M. Kinetic Study and Modeling of the Degradation of Aqueous Ammonium/Ammonia Solutions by Heterogeneous Photocatalysis with TiO2 in a UV-C Pilot Photoreactor. Catalysts. 2022; 12(3):352. https://doi.org/10.3390/catal12030352
Chicago/Turabian StyleGarcía-Prieto, Juan C., Luis A. González-Burciaga, José B. Proal-Nájera, and Manuel García-Roig. 2022. "Kinetic Study and Modeling of the Degradation of Aqueous Ammonium/Ammonia Solutions by Heterogeneous Photocatalysis with TiO2 in a UV-C Pilot Photoreactor" Catalysts 12, no. 3: 352. https://doi.org/10.3390/catal12030352