Insights into a New Formation Mechanism of Robust Cu/SiO2 Catalysts for Low-Temperature Dimethyl Oxalate Hydrogenation Induced by a Chelating Ligand of EDTA
Abstract
:1. Introduction
2. Results
2.1. Structure and Physicochemical Properties of Catalysts
2.2. Evolution of Crystalline Phase and Morphology
2.3. Chemical States of Surface Species
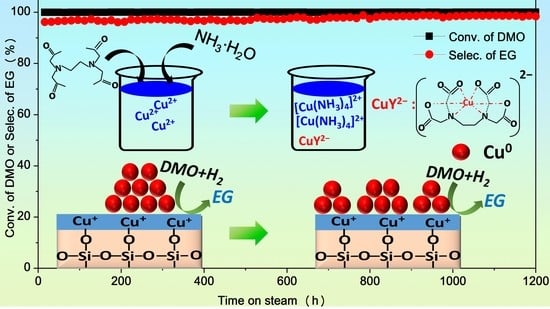
2.4. Catalytic Activity and Stability
3. Discussion
3.1. Interaction between EDTA and Silica Sol
3.2. Effect of EDTA on Surface Copper Species of the Catalysts
3.3. Structure–Performance Relationship
4. Experimental
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, R.P.; Lin, L.; Wang, L.C.; Ding, D.; Zhou, Z.F.; Pan, P.B.; Xu, Z.H.; Liu, J.; Adidharma, H.; Radosz, M.; et al. Perspectives on the active sites and catalyst design for the hydrogenation of dimethyl oxalate. ACS Catal. 2020, 10, 4465–4490. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Market and technology progress of syngas to ethylene glycol. Mod. Chem. Ind. 2017, 37, 5. [Google Scholar]
- Lv, J.; Ma, X.; Wang, Y.; Zhao, Y. High-performance copper-based catalysts for ethylene glycol and ethanol synthesis via ester hydrogenation reactions. Sci. Sin. Chim. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Peng, S.Y.; Zhou, Z.F.; Pan, P.B.; Lin, L.; Qin, Y.Y.; Guo, G.C.; Yao, Y.G. New catalysts for coal to ethylene glycol. Chin. J. Chem. 2017, 35, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yang, Q.C.; Xu, S.M.; Zhu, S.; Zhang, D.W. Technoeconomic and environmental analysis of ethylene glycol production from coal and natural gas compared with oil-based production. J. Clean. Prod. 2020, 273, 123120. [Google Scholar] [CrossRef]
- Ye, R.P.; Wang, X.Y.; Price, C.A.H.; Liu, X.Y.; Yang, Q.H.; Jaroniec, M.; Liu, J. Engineering of yolk/core-shell structured nanoreactors for thermal hydrogenations. Small 2021, 17, e1906250. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.X.; Chen, W.; Wu, P.; Zhu, Z.R.; Li, X.H. Cu-Mg-Zr/SiO2 catalyst for the selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Catal. Sci. Technol. 2018, 8, 2624–2635. [Google Scholar] [CrossRef]
- Vandescheur, F.T.; Staal, L.H. Effects of zinc addition to silica supported copper catalysts for the hydrogenolysis of esters. Appl. Catal. A Gen. 1994, 108, 63–83. [Google Scholar] [CrossRef]
- Ye, C.; Guo, C.; Sun, C.; Zhang, Y. Effect of Mn doping on the activity and stability of Cu–SiO2 catalysts for the hydrogenation of methyl acetate to ethanol. RSC Adv. 2016, 6, 113796–113802. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Li, Q.H.; Zhou, Z.F.; Wang, T.T.; Russell, C.K.; Adidharma, H.; Xu, Z.H.; Yao, Y.G.; Fan, M.H. Recent progress in improving the stability of copper-based catalysts for hydrogenation of carbon-oxygen bonds. Catal. Sci. Technol. 2018, 8, 3428–3449. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Li, H.; Fan, K. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A Gen. 2008, 349, 91–99. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, L.; Ye, R.P.; Sun, M.L.; Yang, J.X.; Li, F.; Yao, Y.G. Mannitol as a novel dopant for Cu/SiO2: A low-cost, environmental and highly stable catalyst for dimethyl oxalate hydrogenation without hydrogen prereduction. J. Catal. 2020, 389, 421–431. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Guo, X.; Dai, W.-L.; Fan, K. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate. J. Catal. 2011, 280, 77–88. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Wang, S.-R.; Zhu, L.-J.; Ge, X.-L.; Li, X.-B.; Luo, Z.-Y. The influence of copper particle dispersion in Cu/SiO2 catalysts on the hydrogenation synthesis of ethylene glycol. Catal. Lett. 2010, 135, 275–281. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Oguchi, H.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Cu2O as active species in the steam reforming of methanol by CuO/ZrO2 catalysts. Appl. Catal. A Gen. 2005, 293, 64–70. [Google Scholar] [CrossRef]
- Ye, R.-P.; Lin, L.; Liu, C.-Q.; Chen, C.-C.; Yao, Y.-G. One-pot synthesis of cyclodextrin-doped Cu-SiO2 catalysts for efficient hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Ye, R.-P.; Lin, L.; Yang, J.-X.; Sun, M.-L.; Li, F.; Li, B.; Yao, Y.-G. A new low-cost and effective method for enhancing the catalytic performance of CuSiO2 catalysts for the synthesis of ethylene glycol via the vapor-phase hydrogenation of dimethyl oxalate by coating the catalysts with dextrin. J. Catal. 2017, 350, 122–132. [Google Scholar] [CrossRef]
- Ryczkowski, J. IR studies of EDTA alkaline salts interaction with the surface of inorganic oxides. Appl. Surf. Sci. 2005, 252, 813–822. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Ye, R.P.; Liao, L.; Reina, T.R.; Liu, J.X.; Chevella, D.; Jin, Y.G.; Fan, M.H.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2021, 285, 119151. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, L.; Ye, R.P.; Huang, L.; Zhu, L.B.; Huang, Y.Y.; Qin, Y.Y.; Yao, Y.G. Construction of Cu-Ce composite oxides by simultaneous ammonia evaporation method to enhance catalytic performance of Ce-Cu/SiO2 catalysts for dimethyl oxalate hydrogenation. Fuel 2021, 290, 120083. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Fan, G.; Li, F. Highly-dispersed copper-based catalysts from Cu-Zn-Al layered double hydroxide precursor for gas-phase hydrogenation of dimethyl oxalate to ethylene glycol. Catal. Lett. 2012, 142, 1121–1127. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Dai, W.-L.; Fan, K. Surface modification of HMS material with silica sol leading to a remarkable enhanced catalytic performance of Cu/SiO2. Appl. Surf. Sci. 2011, 257, 5844–5849. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Modica, V.H.; Ravasio, N. Catalytic selective reduction of NO with ethylene over a series of copper catalysts on amorphous silicas. Appl. Catal. B Environ. 2000, 28, 175–185. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper-catalysts by means of depostion precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Huang, Y.; Ariga, H.; Zheng, X.; Duan, X.; Takakusagi, S.; Asakura, K.; Yuan, Y. Silver-modulated SiO2-supported copper catalysts for selective hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2013, 307, 74–83. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Zhao, G.; Chen, Y.; Zhang, Q.; Chai, R.; Liu, Y.; Lu, Y. Copper-fiber-structured Pd-Au-CuOx: Preparation and catalytic performance in the vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2016, 8, 1065–1073. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, L.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Hydrogenation of dimethyl oxalate to ethylene glycol on a Cu/SiO2/cordierite monolithic catalyst: Enhanced internal mass transfer and stability. AIChE J. 2012, 58, 2798–2809. [Google Scholar] [CrossRef]
- Wang, M.; Yao, D.; Li, A.; Yang, Y.; Lv, J.; Huang, S.; Wang, Y.; Ma, X. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification. Ind. Eng. Chem. Res. 2020, 59, 9414–9422. [Google Scholar] [CrossRef]
- Zaitoun, M.A.; Lin, C.T. Chelating behavior between metal ions and EDTA in sol-gel matrix. J. Phys. Chem. B 1997, 101, 1857–1860. [Google Scholar] [CrossRef]
- Chang, W.; Peng-ran, G.U.O.; Hang-ting, C.; Yong-hong, S.H.U. Evaluation of bioavailability of heavy metals in soils and sediments. Rock Miner. Anal. 2009, 28, 108–112. [Google Scholar]
- Wang, Y.; Yang, W.; Yao, D.; Wang, S.; Xu, Y.; Zhao, Y.; Ma, X. Effect of surface hydroxyl group of ultra-small silica on the chemical states of copper catalyst for dimethyl oxalate hydrogenation. Catal. Today 2020, 350, 127–135. [Google Scholar] [CrossRef]
- Marchi, A.J.; Fierro, J.L.G.; Santamaria, J.; Monzon, A. Dehydrogenation of isopropylic alcohol on a Cu/SiO2 catalyst: A study of the activity evolution and reactivation of the catalyst. Appl. Catal. A Gen. 1996, 142, 375–386. [Google Scholar] [CrossRef]
- Gong, J.L.; Yue, H.R.; Zhao, Y.J.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.P.; Ma, X.B. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Ma, X.G.; Yang, Z.Q.; Liu, X.B.; Tan, X.Z.; Ge, Q.J. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation. RSC Adv. 2015, 5, 37581–37584. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Lv, J.; Ma, X. Facile synthesis of Cu@CeO2 and its catalytic behavior for the hydrogenation of methyl acetate to ethanol. ChemCatChem 2017, 9, 2085–2090. [Google Scholar] [CrossRef]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Ni and/or Ni-Cu alloys supported over SiO2 catalysts synthesized via phyllosilicate structures for steam reforming of biomass tar reaction. Catal. Sci. Technol. 2015, 5, 4398–4409. [Google Scholar] [CrossRef]









| Sample | Cu Loading (wt%) a | SBET (m2g−1) b | Vp (cm3g−1) c | dp (nm) d | SCu (m2g−1) e | Cu Dispersion (%) e | C Loading (wt%) f | dCu (nm) g | dCu2O (nm) g |
|---|---|---|---|---|---|---|---|---|---|
| Cu/SiO2 | 21.6 | 394 | 0.46 | 3.8 | 11.2 | 8.0 | <0.3 | 4.9 | 4.1 |
| 0.04E-Cu/SiO2 | 22.2 | 411 | 0.50 | 4.0 | 12.4 | 8.6 | <0.3 | 4.5 | 3.5 |
| 0.08E-Cu/SiO2 | 22.2 | 429 | 0.52 | 4.2 | 12.8 | 9.3 | <0.3 | - | 2.9 |
| 0.12E-Cu/SiO2 | 18.5 | 400 | 0.46 | 4.0 | 11.1 | 9.3 | <0.3 | - | 3.5 |
| 0.3E-Cu/SiO2 | 14.8 | 354 | 0.47 | 4.6 | 8.2 | 8.5 | <0.3 | - | 3.1 |
| Catalysts | KE (eV) a | AP (eV) b | BE of Cu2p 3/2 (eV) | X Cu+ (%) c | ||
|---|---|---|---|---|---|---|
| Cu+ | Cu0 | Cu+ | Cu0 | |||
| Cu/SiO2 | 913.1 | 916.1 | 1846.1 | 1849.1 | 933.0 | 61.3 |
| 0.04E-Cu/SiO2 | 913.2 | 916.2 | 1846.0 | 1849.0 | 932.8 | 60.5 |
| 0.08E-Cu/SiO2 | 912.7 | 916.0 | 1845.8 | 1849.1 | 933.1 | 63.0 |
| 0.12E-Cu/SiO2 | 913.0 | 916.0 | 1846.1 | 1849.0 | 933.0 | 61.6 |
| 0.3E-Cu/SiO2 | 912.5 | 915.8 | 1845.6 | 1848.9 | 933.1 | 55.8 |
| Sample | 160 °C | 180 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conv. (%) | Selec. (%) | TOF c (h−1) | Conv. (%) | Selec. (%) | |||||||
| EG | EtOH | MG | Other b | EG | EtOH | MG | Other b | ||||
| Cu/SiO2 | 28.80 | 8.43 | 0.02 | 91.32 | 8.66 | 8.62 | 96.34 | 56.58 | 0.05 | 43.05 | 56.9 |
| 0.04E-Cu/SiO2 | 43.44 | 12.54 | 0.03 | 87.22 | 0.21 | 11.75 | 99.76 | 87.32 | 0.15 | 12.10 | 0.43 |
| 0.08E-Cu/SiO2 | 60.90 | 15.10 | 0.02 | 84.70 | 0.18 | 15.95 | 99.99 | 97.70 | 0.50 | 0.78 | 1.02 |
| 0.12E-Cu/SiO2 | 43.60 | 12.93 | 0.04 | 86.80 | 0.23 | 13.17 | 99.98 | 97.78 | 0.61 | 0.65 | 0.96 |
| 0.3E-Cu/SiO2 | 25.86 | 7.04 | 0.05 | 92.64 | 0.27 | 10.57 | 99.24 | 67.89 | 0.06 | 31.79 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Lin, L.; Chen, C.; Ye, R.; Huang, L.; Yang, J.; Zhang, P.; Qin, Y.; Cheng, J.; Yao, Y. Insights into a New Formation Mechanism of Robust Cu/SiO2 Catalysts for Low-Temperature Dimethyl Oxalate Hydrogenation Induced by a Chelating Ligand of EDTA. Catalysts 2022, 12, 320. https://doi.org/10.3390/catal12030320
Li T, Lin L, Chen C, Ye R, Huang L, Yang J, Zhang P, Qin Y, Cheng J, Yao Y. Insights into a New Formation Mechanism of Robust Cu/SiO2 Catalysts for Low-Temperature Dimethyl Oxalate Hydrogenation Induced by a Chelating Ligand of EDTA. Catalysts. 2022; 12(3):320. https://doi.org/10.3390/catal12030320
Chicago/Turabian StyleLi, Tianyou, Ling Lin, Chongchong Chen, Runping Ye, Long Huang, Jinxia Yang, Peng Zhang, Yeyan Qin, Jiankai Cheng, and Yuangen Yao. 2022. "Insights into a New Formation Mechanism of Robust Cu/SiO2 Catalysts for Low-Temperature Dimethyl Oxalate Hydrogenation Induced by a Chelating Ligand of EDTA" Catalysts 12, no. 3: 320. https://doi.org/10.3390/catal12030320