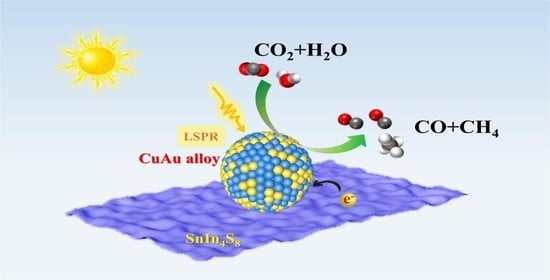
Synergistic Effect in Plasmonic CuAu Alloys as Co-Catalyst on SnIn4S8 for Boosted Solar-Driven CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zu, X.; Zhao, Y.; Li, X.; Chen, R.; Shao, W.; Wang, Z.; Hu, J.; Zhu, J.; Pan, Y.; Sun, Y.; et al. Ultrastable and Efficient Visible-light-driven CO2 Reduction Triggered by Regenerative Oxygen-Vacancies in Bi2O2CO3 Nanosheets. Angew. Chem. Int. Ed. 2021, 60, 13840–13846. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, C.; Yang, Z.; Wei, J. Encapsulated CdSe/CdS nanorods in double-shelled porous nanocomposites for efficient photocatalytic CO2 reduction. Nat. Commun. 2022, 13, 6466. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, T. Recent advances in zinc chalcogenide-based nanocatalysts for photocatalytic reduction of CO2. J. Mater. Chem. A 2021, 9, 23364–23381. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4-In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Wang, J.; Bo, T.; Shao, B.; Zhang, Y.; Jia, L.; Tan, X.; Zhou, W.; Yu, T. Effect of S vacancy in Cu3SnS4 on high selectivity and activity of photocatalytic CO2 reduction. Appl. Catal. B. 2021, 297, 120498. [Google Scholar] [CrossRef]
- Yu, H.; Chen, F.; Li, X.; Huang, H.; Zhang, Q.; Su, S.; Wang, K.; Mao, E.; Mei, B.; Mul, G.; et al. Synergy of ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction. Nat. Commun. 2021, 12, 4594. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Nanostructured Metal Sulfides: Classification, Modification Strategy, and Solar-Driven CO2 Reduction Application. Adv. Funct. Mater. 2020, 31, 2008008. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar]
- Yan, T.; Li, L.; Li, G.; Wang, Y.; Hu, W.; Guan, X. Porous SnIn4S8 microspheres in a new polymorph that promotes dyes degradation under visible light irradiation. J. Hazard. Mater. 2011, 186, 272–279. [Google Scholar] [CrossRef]
- Shen, C.; Chen, Y.; Xu, X.; Li, X.; Wen, X.; Liu, Z.; Xing, R.; Guo, H.; Fei, Z. Efficient photocatalytic H2 evolution and Cr(VI) reduction under visible light using a novel Z-scheme SnIn4S8/CeO2 heterojunction photocatalysts. J. Hazard. Mater. 2021, 416, 126217. [Google Scholar] [CrossRef]
- Chen, C.C.; Shaya, J.; Polychronopoulou, K.; Golovko, V.B.; Tesana, S.; Wang, S.Y.; Lu, C.S. Photocatalytic Degradation of Ethiofencarb by a Visible Light-Driven SnIn4S8 Photocatalyst. Nanomaterials 2021, 11, 1325. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, M.; Wang, Y.; Zhang, A.; Zhao, X.; Zeng, G.; Deng, F. Bandgap engineering of hierarchical network-like SnIn4S8 microspheres through preparation temperature for excellent photocatalytic performance and high stability. Green Energy Environ. 2019, 4, 264–269. [Google Scholar] [CrossRef]
- Han, X.; Xu, D.; An, L.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Ni-Mo nanoparticles as co-catalyst for drastically enhanced photocatalytic hydrogen production activity over g-C3N4. Appl. Catal. B 2019, 243, 136–144. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Shi, X.; Zhang, Q.; Song, Q.; Zhou, Y.; Jiang, D. PtAg alloys as an efficient co-catalyst for CO2 deep photoreduction with H2O: Synergistic effects of Pt and Ag. Appl. Surf. Sci. 2022, 598, 153843. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, Y.; Zhang, Q.; Song, Q.; Zhou, C.; Shi, X.; Li, D. Synergistic Integration of AuCu Co-Catalyst with Oxygen Vacancies on TiO2 for Efficient Photocatalytic Conversion of CO2 to CH4. ACS Appl. Mater. Interfaces 2021, 13, 46772–46782. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, F.; Wang, D.; Cai, X.; Meng, C.; Xie, H.; Zhang, J.; Bai, S. Lattice Engineering on Metal Cocatalysts for Enhanced Photocatalytic Reduction of CO2 into CH4. ChemSusChem 2018, 11, 3524–3533. [Google Scholar] [CrossRef]
- Li, W.; Chu, X.; Wang, F.; Dang, Y.; Liu, X.; Wang, X.; Wang, C. Enhanced cocatalyst-support interaction and promoted electron transfer of 3D porous g-C3N4/GO-M (Au, Pd, Pt) composite catalysts for hydrogen evolution. Appl. Catal. B 2021, 288, 120034. [Google Scholar] [CrossRef]
- Haider, R.S.; Wang, S.; Gao, Y.; Malik, A.S.; Ta, N.; Li, H.; Zeng, B.; Dupuis, M.; Fan, F.; Li, C. Boosting photocatalytic water oxidation by surface plasmon resonance of AgxAu1−x alloy nanoparticles. Nano Energy 2021, 87, 106189. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Shi, Q.; Xia, H.; Du, D.; Lin, Y. Highly branched PtCu bimetallic alloy nanodendrites with superior electrocatalytic activities for oxygen reduction reactions. Nanoscale 2016, 8, 5076–5081. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Kim, W.D.; Lee, S.; Lee, K.; Bae, W.K.; Moon, J.H.; Lee, S.; Lee, D.C. Low-coordinated surface atoms of CuPt alloy cocatalysts on TiO2 for enhanced photocatalytic conversion of CO2. Nanoscale 2016, 8, 10043–10048. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Dong, X.; Chen, P.; Geng, Q.; Wang, H.; Li, J.; Zhou, Y.; Dong, F. Synergistic Effect of Cu Single Atoms and Au-Cu Alloy Nanoparticles on TiO2 for Efficient CO2 Photoreduction. ACS Nano 2021, 15, 14453–14464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Q.; Li, T.; Ren, Q.; Zhong, S.; Zhao, Y.; Bai, S. Vacancy engineering of AuCu cocatalysts for improving the photocatalytic conversion of CO2 to CH4. J. Mater. Chem. A 2019, 7, 27007–27015. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, H.; Chen, J.; Wu, M.; Leung, D.Y.C.; Grimes, C.A.; Lu, Z.; Xu, Z.; Feng, S.-P. Synergistic effects of Pd-Ag bimetals and g-C3N4 photocatalysts for selective and efficient conversion of gaseous CO2. J. Power Sources 2020, 466, 228306. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, J.; Lv, Y.; Fu, X.; Jian, Y.; Zhang, W.; Wang, Y.; Sun, H.; Wang, X.; Long, J.; et al. Low-crystalline PdCu alloy on large-area ultrathin 2D carbon nitride nanosheets for efficient photocatalytic Suzuki coupling. Appl. Catal. B 2022, 300, 120756. [Google Scholar] [CrossRef]
- Cai, X.; Wang, A.; Wang, J.; Wang, R.; Zhong, S.; Zhao, Y.; Wu, L.; Chen, J.; Bai, S. Order engineering on the lattice of intermetallic PdCu co-catalysts for boosting the photocatalytic conversion of CO2 into CH4. J. Mater. Chem. A 2018, 6, 17444–17456. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Liu, Y.; Chen, S.; Zheng, X.; Gao, C.; He, C.; Chen, N.; Qi, Z.; Song, L.; et al. Isolation of Cu Atoms in Pd Lattice: Forming Highly Selective Sites for Photocatalytic Conversion of CO2 to CH4. J. Am. Chem. Soc. 2017, 139, 4486–4492. [Google Scholar] [CrossRef]
- Bhunia, K.; Chandra, M.; Khilari, S.; Pradhan, D. Bimetallic PtAu Alloy Nanoparticles-Integrated g-C3N4 Hybrid as an Efficient Photocatalyst for Water-to-Hydrogen Conversion. ACS Appl. Mater. Interfaces 2019, 11, 478–488. [Google Scholar] [CrossRef]
- Yan, P.C.; Jin, Y.C.; Xu, L.; Mo, Z.; Qian, J.C.; Chen, F.; Yuan, J.J.; Xu, H.; Li, H.N. Enhanced photoelectrochemical aptasensing triggered by nitrogen deficiency and cyano group simultaneously engineered 2D carbon nitride for sensitively monitoring atrazine. Biosens. Bioelectron. 2022, 206, 114144. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Dao, T.D.; Liu, Y.; Zhou, W.; Liu, L.; Meng, X.; Nagao, T.; Ye, J. Design of PdAu alloy plasmonic nanoparticles for improved catalytic performance in CO2 reduction with visible light irradiation. Nano Energy 2016, 26, 398–404. [Google Scholar] [CrossRef]
- Shi, L.Z.; Liu, H.M.; Ning, S.B.; Ye, J.H. Localized surface plasmon resonance effect enhanced Cu/TiO2 core-shell catalyst for boosting CO2 hydrogenation reaction. Catal. Sci. Technol. 2022, 12, 6155–6162. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Dao, T.D.; Zhang, H.; Li, P.; Chang, K.; Wang, T.; Li, M.; Nagao, T.; Ye, J. Conversion of Carbon Dioxide by Methane Reforming under Visible-Light Irradiation: Surface-Plasmon-Mediated Nonpolar Molecule Activation. Angew. Chem. Int. Ed. Engl. 2015, 54, 11545–11549. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wu, Y.; Rhimi, B.; Qin, J.; Huang, Y.; Yuan, M.; Wang, C. Oxygen-doping of ZnIn2S4 nanosheets towards boosted photocatalytic CO2 reduction. J. Energy Chem. 2021, 57, 1–9. [Google Scholar] [CrossRef]
- Lang, Q.; Yang, Y.; Zhu, Y.; Hu, W.; Jiang, W.; Zhong, S.; Gong, P.; Teng, B.; Zhao, L.; Bai, S. High-index facet engineering of PtCu cocatalysts for superior photocatalytic reduction of CO2 to CH4. J. Mater. Chem. A 2017, 5, 6686–6694. [Google Scholar] [CrossRef]
- Cai, X.; Wang, F.; Wang, R.; Xi, Y.; Wang, A.; Wang, J.; Teng, B.; Bai, S. Synergism of surface strain and interfacial polarization on Pd@Au core–shell cocatalysts for highly efficient photocatalytic CO2 reduction over TiO2. J. Mater. Chem. A 2020, 8, 7350–7359. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Mo, Z.; Wu, G.; Yan, P.; Zhu, X.; Qian, J.; Lei, Y.; Xu, L.; Xu, H.; Li, H. Engineering oxygen into ultrathin graphitic carbon nitride: Synergistic improvement of electron reduction and charge carrier dynamics for efficient photocatalysis. Mater. Today Chem. 2022, 25, 100956. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.P.; Ma, C.C.; Zhu, Z.; Song, X.H.; Li, X.Y.; Wang, H.Q.; Huo, P.W.; Chen, X.B. Construction of a multi-interfacial-electron transfer scheme for efficient CO2 photoreduction: A case study using CdIn2S4 micro-flower spheres modified with Au nanoparticles and reduced graphene oxide. J. Mater. Chem. A 2020, 8, 18707–18714. [Google Scholar] [CrossRef]
- Tian, J.; Zhong, K.; Zhu, X.; Yang, J.; Mo, Z.; Liu, J.; Dai, J.; She, Y.; Song, Y.; Li, H.; et al. Highly exposed active sites of Au nanoclusters for photocatalytic CO2 reduction. Chem. Eng. J. 2023, 451, 138392. [Google Scholar]
- Han, D.; Bao, Z.; Xing, H.; Yang, Y.; Ren, Q.; Zhang, Z. Fabrication of plasmonic Au-Pd alloy nanoparticles for photocatalytic Suzuki-Miyaura reactions under ambient conditions. Nanoscale 2017, 9, 6026–6032. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; Zhou, G.; Li, Q.; Wang, C.; Hua, Y.; She, Y.; Xu, H.; Li, H. Plasma-induced defect engineering: Boosted the reverse water gas shift reaction performance with electron trap. J. Colloid Interface Sci. 2020, 580, 814–821. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Yu, Q.; He, M.; Zhang, W.; Mo, Z.; Yuan, J.; She, Y.; Xu, H.; Li, H. Multidimensional In2O3/In2S3 heterojunction with lattice distortion for CO2 photoconversion. Chin. J. Catal. 2022, 43, 1286–1294. [Google Scholar] [CrossRef]
- Yang, J.; Jing, L.; Zhu, X.; Zhang, W.; Deng, J.; She, Y.; Nie, K.; Wei, Y.; Li, H.; Xu, H. Modulating electronic structure of lattice O-modified orange polymeric carbon nitrogen to promote photocatalytic CO2 conversion. Appl. Catal. B 2023, 320, 122005. [Google Scholar] [CrossRef]
- Huang, J.; Mensi, M.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural Sensitivities in Bimetallic Catalysts for Electrochemical CO2 Reduction Revealed by Ag-Cu Nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Ji, H.; He, M.; Song, Y.; Zhang, W.; Yuan, J.; She, X.; She, Y.; Li, H.; et al. Construction of S-Co-S internal electron transport bridges in Co-doped CuInS2 for enhancing photocatalytic CO2 reduction. Mater. Today Chem. 2022, 26, 101078. [Google Scholar] [CrossRef]
- Qiu, B.; Huang, P.; Lian, C.; Ma, Y.; Xing, M.; Liu, H.; Zhang, J. Realization of all-in-one hydrogen-evolving photocatalysts via selective atomic substitution. Appl. Catal. B 2021, 298, 120518. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, Y.F.; Xu, J.Q.; Shao, Y.J.; Wu, J.; Xu, X.L.; Pan, Y.; Ju, H.X.; Zhu, J.F.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Zhu, X.; Yuan, J.; Zhou, M.; She, X.; Yu, Q.; Song, Y.; She, Y.; Hua, Y.; et al. Exploring deep effects of atomic vacancies on activating CO2 photoreduction via rationally designing indium oxide photocatalysts. Chem. Eng. J. 2021, 422, 129888. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.; Mo, Z.; Yi, J.; Yan, J.; Deng, J.; Xu, Y.; She, Y.; Qian, J.; Xu, H.; et al. A multidimensional In2S3-CuInS2 heterostructure for photocatalytic carbon dioxide reduction. Inorg. Chem. Front. 2018, 5, 3163–3169. [Google Scholar]
- Zhang, Z.; Cao, Y.; Zhang, F.; Li, W.; Li, Y.; Yu, H.; Wang, M.; Yu, H. Tungsten oxide quantum dots deposited onto ultrathin CdIn2S4 nanosheets for efficient S-scheme photocatalytic CO2 reduction via cascade charge transfer. Chem. Eng. J. 2022, 428, 131218. [Google Scholar] [CrossRef]
- Han, Q.; Li, L.; Gao, W.; Shen, Y.; Wang, L.; Zhang, Y.; Wang, X.; Shen, Q.; Xiong, Y.; Zhou, Y.; et al. Elegant Construction of ZnIn2S4/BiVO4 Hierarchical Heterostructures as Direct Z-Scheme Photocatalysts for Efficient CO2 Photoreduction. ACS Appl. Mater. Interfaces 2021, 13, 15092–15100. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Near-infrared absorbing 2D/3D ZnIn2S4/N-doped graphene photocatalyst for highly efficient CO2 capture and photocatalytic reduction. Sci. China Mater. 2020, 63, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Hu, J.; Hu, X.; Zhang, W.; Ma, X.; Wang, H. Rational Construction of Z-Scheme CuInS2/Au/g-C3N4 Heterostructure: Experimental Results and Theoretical Calculation. ChemCatChem 2019, 11, 6372–6383. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yang, J.; Yang, K.; Zhu, X.; Zhong, K.; Zhang, M.; Ji, H.; He, M.; Li, H.; Xu, H. Synergistic Effect in Plasmonic CuAu Alloys as Co-Catalyst on SnIn4S8 for Boosted Solar-Driven CO2 Reduction. Catalysts 2022, 12, 1588. https://doi.org/10.3390/catal12121588
Yang Z, Yang J, Yang K, Zhu X, Zhong K, Zhang M, Ji H, He M, Li H, Xu H. Synergistic Effect in Plasmonic CuAu Alloys as Co-Catalyst on SnIn4S8 for Boosted Solar-Driven CO2 Reduction. Catalysts. 2022; 12(12):1588. https://doi.org/10.3390/catal12121588
Chicago/Turabian StyleYang, Zhengrui, Jinman Yang, Kefen Yang, Xingwang Zhu, Kang Zhong, Ming Zhang, Haiyan Ji, Minqiang He, Huaming Li, and Hui Xu. 2022. "Synergistic Effect in Plasmonic CuAu Alloys as Co-Catalyst on SnIn4S8 for Boosted Solar-Driven CO2 Reduction" Catalysts 12, no. 12: 1588. https://doi.org/10.3390/catal12121588