Augmenting the Photocatalytic Performance of Direct Z-Scheme Bi2O3/g-C3N4 Nanocomposite
Abstract
:1. Introduction
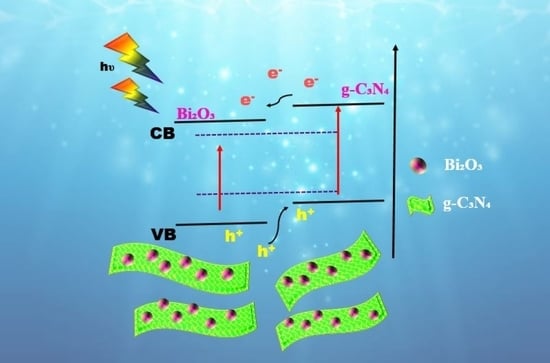
2. Results and Discussion
Evaluation of Photocatalytic Activity
3. Materials and Methods
Photocatalysis Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuvaneswari, K.; Bharathi, R.D.; Pazhanivel, T. Silk fibroin linked Zn/Cd-doped SnO2 nanoparticles to purify the organically polluted water. Mater. Res. Express. 2018, 5, 024004. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.U.; Riaz, M.; Iqbal, F. Dual S-scheme heterojunction ZnO–V2O5–WO3 nanocomposite with enhanced photocatalytic and antimicrobial activity. Mater. Chem. Phys. 2021, 263, 124372. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.U.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Palanisamy, G.; Pazhanivel, T.; Bharathi, G.; Nataraj, D. Photocatalytic Performance on Visible Light Induced ZnS QDs-MgAl Layered Double Hydroxides Hybrids for Methylene Blue Dye Degradation. Chem. Sel. 2018, 3, 13419–13426. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped g-C3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365–28375. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, B.; Mudisoodum Perumal, S.; Maiyalagan, T.; Jayaraman, V.; Ayyappan, C.; Alagiri, M. Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl. Surf. Sci. 2019, 498, 143807. [Google Scholar] [CrossRef]
- Palanivel, B.; Ayappan, C.; Jayaraman, V.; Chidambaram, S.; Maheshwaran, R.; Alagiri, M. Inverse spinel NiFe2O4 deposited g-C3N4 nanosheet for enhanced visible light photocatalytic activity. Mater. Sci. Semicond. 2019, 100, 87–97. [Google Scholar] [CrossRef]
- Li, B.; Nengzi, L.C.; Guo, R.; Cui, Y.; Zhang, Y.; Cheng, X. Novel synthesis of Z-scheme α-Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance: Influence of calcination temperature. Chin. Chem. Lett. 2020, 31, 2705–2711. [Google Scholar] [CrossRef]
- Fan, G.; Ma, Z.; Li, X.; Deng, L. Coupling of Bi2O3 nanoparticles with g-C3N4 for enhanced photocatalytic degradation of methylene blue. Ceram. Int. 2021, 47, 5758–5766. [Google Scholar] [CrossRef]
- Palanive, B.; Alagiri, M. Construction of rGO supported integrative NiFe2O4/g-C3N4 nanocomposite: Role of charge transfer for boosting the OH• radical production to enhance the photo-Fenton degradation. ChemistrySelect 2020, 5, 9765–9775. [Google Scholar] [CrossRef]
- Matheswaran, P.; Thangavelu, P.; Palanivel, B. Carbon dot sensitized integrative g-C3N4/AgCl Hybrids: An synergetic interaction for enhanced visible light driven photocatalytic process. Adv. Powder Technol. 2019, 30, 1715–1723. [Google Scholar] [CrossRef]
- Matheswaran, P.; Karuppiah, P.; Chen, S.M.; Thangavelu, P.; Ganapathi, B. Fabrication of g-C3N4 Nanomesh-Anchored Amorphous NiCoP2O7: Tuned Cycling Life and the Dynamic Behavior of a Hybrid Capacitor. ACS Omega 2018, 3, 18694–18704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, H.; Liu, W.; Chen, H.; Qu, Y.; Lin, Z. The construction of the heterostructural Bi2O3/g-C3N4 composites with an enhanced photocatalytic activity. Nano 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Xu, D.; Zhang, X.; Shen, M. Enhanced photocatalytic activity of direct Z-scheme Bi2O3/g-C3N4 composites via facile one-step fabrication. J. Mater. Res. 2018, 33, 1391–1400. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, Z.; Tang, H.; Jiang, C. Fabrication of flower-like direct Z-scheme β-Bi2O3/g-C3N4 photocatalyst with enhanced visible light photoactivity for Rhodamine B degradation. Appl. Surf. Sci. 2018, 436, 162–171. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Guo, R.; Zhang, H.; Li, B.; Xie, M.; Cheng, Q.; Cheng, X. Construction of Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance and mechanism. Sep. Purif. Technol. 2018, 203, 301–309. [Google Scholar] [CrossRef]
- Zhu, Z.; Huo, P.; Lu, Z.; Yan, Y.; Liu, Z.; Shi, W.; Li, C.; Dong, H. Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator. Chem. Eng. J. 2018, 331, 615–625. [Google Scholar] [CrossRef]
- Palanivel, B.; Shkir, M.; Alshahrani, T.; Mani, A. Novel NiFe2O4 deposited S-doped g-C3N4 nanorod: Visible-light-driven heterojunction for photo-Fenton like tetracycline degradation. Diam. Relat. Mater. 2021, 112, 108148. [Google Scholar] [CrossRef]
- De Almeida, M.F.; Bellato, C.R.; Mounteer, A.H.; Ferreira, S.O.; Milagres, J.L.; Miranda, L.D.L. Enhanced photocatalytic activity of TiO2—Impregnated with MgZnAl mixed oxides obtained from layered double hydroxides for phenol degradation. Appl. Surf. Sci. 2015, 357, 1765–1775. [Google Scholar] [CrossRef]
- Miao, X.; Ji, Z.; Wu, J.; Shen, X.; Wang, J.; Kong, L.; Liu, M.; Song, C. g-C3N4/AgBr nanocomposite decorated with carbon dots as a highly efficient visible-light-driven photocatalyst. J. Colloid Interface Sci. 2017, 502, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, B.; Mani, A. Conversion of a Type-II to a Z-Scheme heterojunction by intercalation of a 0D electron mediator between the integrative NiFe2O4/g-C3N4 composite nanoparticles: Boosting the radical production for photo-Fenton degradation. ACS Omega 2020, 5, 19747–19759. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, B.; Jayaraman, V.; Ayyappan, C.; Alagiri, M. Magnetic binary metal oxide intercalated g-C3N4: Energy band tuned pn heterojunction towards Z-scheme photo-Fenton phenol reduction and mixed dye degradation. J. Water Process Eng. 2019, 32, 100968. [Google Scholar] [CrossRef]
- Palanivel, B.; Hossain, M.S.; Macadangdang, R.R., Jr.; Ayappan, C.; Krishnan, V.; Marnadu, R.; Kalaivani, T.; Alharthi, F.A.; Sreedevi, G. Activation of persulfate for improved naproxen degradation using FeCo2O4@g-C3N4 heterojunction photocatalysts. ACS Omega 2021, 6, 34563–34571. [Google Scholar] [CrossRef]
- Yan, X.; Xu, R.; Guo, J.; Cai, X.; Chen, D.; Huang, L.; Xiong, Y.; Tan, S. Enhanced photocatalytic activity of Cu2O/g-C3N4 heterojunction coupled with reduced graphene oxide three-dimensional aerogel photocatalysis. Mater. Res. Bull. 2017, 96, 18–27. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Z.; Wang, F.; Qi, Y.; Zhang, W.; Wang, C. State-of-the-art and prospects of Zn-containing layered double hydroxides (Zn-LDH)-based materials for photocatalytic water remediation. Chemosphere 2021, 278, 130367. [Google Scholar] [CrossRef]
- Liu, X.; Ji, X.; Liu, M.; Liu, N.; Tao, Z.; Dai, Q.; Wei, L.; Li, C.; Zhang, X.; Wang, B. High-performance ge quantum dot decorated graphene/zinc-oxide heterostructure infrared photodetector. ACS Appl. Mater. Interfaces 2015, 7, 2452–2458. [Google Scholar] [CrossRef]
- George, A.; Raj, D.M.A.; Raj, A.D.; Nguyen, B.-S.; Phan, T.-P.; Pazhanivel, T.; Sivashanmugan, K.; Josephine, R.; Irudayaraj, A.A.; Arumugam, J.; et al. Morphologically tailored CuO nanostructures toward visible-light-driven photocatalysis. Mater. Lett. 2020, 281, 128603. [Google Scholar] [CrossRef]








| No. | Catalyst | Pollutant (ppm) | Irradiation Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| 1 | ZnO-V2O5-WO3 | MB (10 ppm) | 80 min | 99.8% | [2] |
| 2 | PANI-CeO2-Fe2O3-NiO | MB (5 ppm) | 50 min | 99% | [3] |
| 3 | Bi2O3/g-C3N4 | Phenol (10 ppm) | 150 min | >70% | [14] |
| 4 | Bi2O3/g-C3N4 | MB (10 ppm) | 120 min | >90% | [15] |
| 5 | Bi2O3/g-C3N4 | Rh B (10 ppm) | 80 min | 98% | [18] |
| 6 | Bi2O3/g-C3N4 | Amido Black (10 ppm) | 120 min | 88.71% | [19] |
| 7 | NiFe2O4/CD/g-C3N4 | Rh B (10 ppm) | 120 min | 97% | [21] |
| 8 | Bi2O3/g-C3N4 | MB (10 ppm) | 120 min | 91.2% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalakshmi, K.; Ranjith, R.; Thangavelu, P.; Priyadharshini, M.; Palanivel, B.; Manthrammel, M.A.; Shkir, M.; Diravidamani, B. Augmenting the Photocatalytic Performance of Direct Z-Scheme Bi2O3/g-C3N4 Nanocomposite. Catalysts 2022, 12, 1544. https://doi.org/10.3390/catal12121544
Mahalakshmi K, Ranjith R, Thangavelu P, Priyadharshini M, Palanivel B, Manthrammel MA, Shkir M, Diravidamani B. Augmenting the Photocatalytic Performance of Direct Z-Scheme Bi2O3/g-C3N4 Nanocomposite. Catalysts. 2022; 12(12):1544. https://doi.org/10.3390/catal12121544
Chicago/Turabian StyleMahalakshmi, Krishnasamy, Rajendran Ranjith, Pazhanivel Thangavelu, Matheshwaran Priyadharshini, Baskaran Palanivel, Mohamed Aslam Manthrammel, Mohd Shkir, and Barathi Diravidamani. 2022. "Augmenting the Photocatalytic Performance of Direct Z-Scheme Bi2O3/g-C3N4 Nanocomposite" Catalysts 12, no. 12: 1544. https://doi.org/10.3390/catal12121544