Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion
Abstract
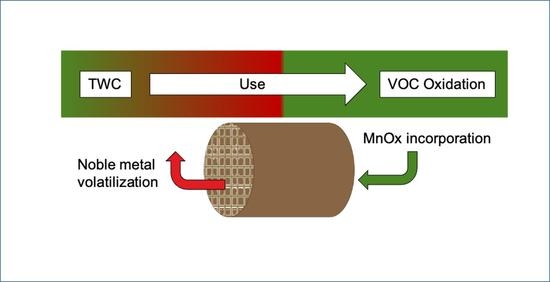
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Monolithic Samples
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control: Commercial Technology, 2nd ed.; Wiley Inter-Science: New York, NY, USA, 2002. [Google Scholar]
- Farrauto, R.J.; Heck, R.M. Catalytic converters. Catal. Today 1999, 51, 351–360. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Gatica, J.M.; Pérez Omil, J.A.; Pintado, J.M. Characterisation of three-way automotive aftertreatment catalysts and related model systems. Top. Catal. 2004, 28, 31–45. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Shim, W.-G.; Jung, S.-C.; Seo, S.-G.; Kim, S.C. Evaluation of regeneration of spent three-way catalysts for catalytic oxidation of aromatic hydrocarbons. Catal. Today 2011, 164, 500–506. [Google Scholar] [CrossRef]
- Angelidis, T.N.; Koutlemani, M.M.; Sklavounos, S.A.; Lioutas, C.B.; Voulgaropoulos, A.; Papadakis, V.G.; Emons, H. Causes of deactivation and an effort to regenerate a commercial spent three-way catalyst. Stud. Surf. Sci. Catal. 1998, 116, 155–164. [Google Scholar]
- Birgersson, H.; Eriksson, L.; Boutonnet, M.; Järås, S. Thermal gas treatment to regenerate spent automotive three-way exhaust gas catalysts (TWC). Appl. Catal. B Environ. 2004, 54, 193–200. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J. Environ. Manag. 2022, 305, 114383. [Google Scholar] [CrossRef] [PubMed]
- Rincón, J.; Asencio, I.; Camarillo, R.; Martín, A. Reciclado de catalizadores de automóviles. Ing. Química 2008, 455, 182–189. [Google Scholar]
- Yoo, J.S. Metal recovery and rejuvenation of metal-loaded spent catalysts. Catal. Today 1998, 44, 27–46. [Google Scholar] [CrossRef]
- Alekseeva, T.Y.; Karpov, Y.A.; Dal’nova, O.A.; Es’kina, V.V.; Baranovskaya, V.B.; Gorbatova, L.D. Current state and problems of analytical control of spent automobile catalysts (Review). Inorganic Mater. 2018, 54, 1421–1429. [Google Scholar] [CrossRef]
- Yoshimura, A.; Matsuno, Y. A fundamental study of platinum recovery from spent auto-catalyst using aqua regia. J. Japan Inst. Met. Mater. 2019, 83, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, S.; Tu, G.; Xiao, F. A novel method for extraction of platinum from spent automotive catalyst: Utilization of spent fluid catalytic cracking catalyst as flux. Environ. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total Environ. 2022, 802, 149830. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.; Panou, M.; Panias, D. Single-step hydrometallurgical method for the platinum group metals leaching from commercial spent automotive catalysts. J. Sustain. Metall. 2020, 6, 259–268. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Giulinao, M.; Kourtelesis, M.; Nicol, G.; Polyzou, E.; Parussa, F.; Yakoumis, I.; Sgroi, M.F. First of its kind automotive catalyst prepared by recycled PGMs-Catalytic performance. Catalysts 2021, 11, 942. [Google Scholar] [CrossRef]
- Gombac, V.; Montini, T.; Falqui, A.; Loche, D.; Prato, M.; Genovese, A.; Mercuri, M.L.; Serpe, A.; Fornasiero, P.; Deplano, P. From trash to resource: Recovered-Pd from spent three-way catalysts as a precursor of an effective photo-catalyst for H2 production. Green Chem. 2016, 18, 2745–2752. [Google Scholar] [CrossRef] [Green Version]
- Trimm, D.L. The regeneration or disposal of deactivated heterogeneous catalysts. Appl. Catal. A Gen. 2001, 212, 153–160. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Shao, Y.; Zhang, G.; Ou, L.; Lu, Y. Research on the recycling of valuable metals in spent Al2O3-based catalyst. Miner. Eng. 2006, 19, 94–97. [Google Scholar] [CrossRef]
- Kim, S.C.; Nahm, S.W.; Shim, W.G.; Lee, J.W.; Moon, H. Influence of physicochemical treatments on spent palladium based catalyst for catalytic oxidation of VOCs. J. Hazard. Mater. 2007, 141, 305–314. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Utilization of spent catalysts for the removal of VOCs. J. Korean Ind. Eng. Chem. 2007, 18, 303–313. [Google Scholar]
- Gatica, J.M.; Castiglioni, J.; de los Santos, C.; Yeste, M.P.; Cifredo, G.; Torres, M.; Vidal, H. Use of pillared clays in the preparation of washcoated clay honeycomb monoliths as support of manganese catalysts for the total oxidation of VOCs. Catal. Today 2017, 296, 84–94. [Google Scholar] [CrossRef]
- De los Santos, C.; Vidal, H.; Gatica, J.M.; Yeste, M.P.; Cifredo, G.; Castiglioni, J. Optimized preparation of washcoated clay honeycomb monoliths as support of manganese catalysts for acetone total combustion. Micropor. Mesopor. Mater. 2021, 310, 110651. [Google Scholar] [CrossRef]
- Azalim, S.; Brahmi., R.; Agunaou., M.; Beaurain., A.; Giraudon., J.-M.; Lamonier., J.-F. Washcoating of cordierite honeycomb with Ce–Zr–Mn mixed oxides for VOC catalytic oxidation. Chem. Eng. J. 2013, 223, 536–546. [Google Scholar] [CrossRef]
- Touati, H.; Valange, S.; Reinholdt, M.; Batiot-Dupeyrat, C.; Clacens, J.-M.; Tatibouët, J.-M. Low temperature catalytic oxidation of ethanol using ozone over manganese oxide-based catalysts in powdered and monolithic forms. Catalysts 2022, 12, 172. [Google Scholar] [CrossRef]
- Hao, C.; Huang, W.; Zhang, J.; Wu, J.; Yue, Y.; Qian, G. Producing a monolithic catalyst by manganese slag and its industrial application in catalytic oxidization of volatile organic compounds. J. Environ. Chem. Eng. 2021, 9, 106145. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Optimization of the preparation conditions of cordierite honeycomb monoliths washcoated with cryptomelane-type manganese oxide for VOC oxidation. Environ. Technol. 2021, 42, 2504–2515. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Figueiredo, J.L.; Sanz, O.; Montes, M.; Pereira, M.F.R. Preparation of ceramic and metallic monoliths coated with cryptomelane as catalysts for VOC abatement. Chem. Eng. J. 2020, 382, 122923. [Google Scholar] [CrossRef]
- Fu, K.; Su, Y.; Yang, L.; Song, C.; Ma, D.; Lv, X.; Han, R.; Liu, Q. Pt loaded manganese oxide nanoarray-based monolithic catalysts for catalytic oxidation of acetone. Chem. Eng. J. 2022, 432, 134397. [Google Scholar] [CrossRef]
- Nowicki, B.; Hetper, J. A monolithic manganese catalyst for removing pollutants from air. Przemiysl Chem. 2004, 83, 444–447. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Angelidis, T.N.; Sklavounos, S.A. A SEM-EDS study of new and used automotive catalysts. Appl. Catal. A Gen. 1995, 133, 121–132. [Google Scholar] [CrossRef]
- Chen, J.-C.; Huang, J.-J. Regeneration of spent catalysts by H2O2 chemical treatment. Apcbee Procedia 2013, 5, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Christou, S.Y.; Birgersson, H.; Efstathiou, A.M. Reactivation of severely aged commercial three-way catalysts by washing with weak EDTA and oxalic acid solutions. Appl. Catal. B Environ. 2007, 71, 185–198. [Google Scholar] [CrossRef]
- Neyerts, C.A.; Banús, E.D.; Miró, E.E.; Querini, C.A. Potassium-promoted Ce0.65Zr0.35O2 monolithic catalysts for diesel soot combustion. Chem. Eng. J. 2014, 248, 394–405. [Google Scholar] [CrossRef]







| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) |
|---|---|---|
| M | 4.7 | 0.037 |
| RM | 4.2 | 0.029 |
| Mn/RM | 3.3 | 0.010 |
| Mn2/RM | 4.6 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De los Santos, C.; Gatica, J.M.; Castiglioni, J.; Vidal, H. Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion. Catalysts 2022, 12, 1514. https://doi.org/10.3390/catal12121514
De los Santos C, Gatica JM, Castiglioni J, Vidal H. Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion. Catalysts. 2022; 12(12):1514. https://doi.org/10.3390/catal12121514
Chicago/Turabian StyleDe los Santos, Carolina, José Manuel Gatica, Jorge Castiglioni, and Hilario Vidal. 2022. "Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion" Catalysts 12, no. 12: 1514. https://doi.org/10.3390/catal12121514