Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase
Abstract
:1. Introduction
2. Results
2.1. Catalysts Characterization
2.2. Characterization of Radicals Intermediates Using EPR
2.3. Formation of H2O2 during the Photocatalytic Reaction of HCOOH and Phenol
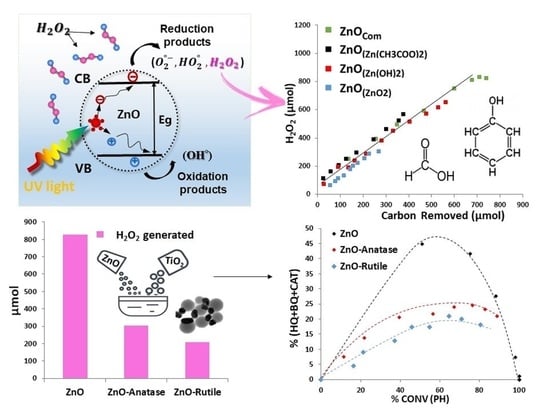
2.3.1. Comparison of the Formation of H2O2 from FA and Ph in the Presence of ZnO-Com
2.3.2. Influence of the Nature of ZnO Samples on H2O2 Formation
2.4. Photocatalytic Decomposition of H2O2
3. Materials and Methods
3.1. Photocatalysts
3.2. Characterization
3.3. Photocatalytic Experiments and Analytical Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced Oxidation Processes (AOP) for Water Purification and Recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A Review of Imperative Technologies for Wastewater Treatment I: Oxidation Technologies at Ambient Conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Mercier, G.; Blais, J.-F. Procédés d’oxydation Avancée Dans Le Traitement Des Eaux et Des Effluents Industriels: Application à La Dégradation Des Polluants Réfractaires. Rev. Des Sci. L’eau/J. Water Sci. 2009, 22, 535–564. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation-A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Lawless, D.; Serpone, N.; Meisel, D. Role of Hydroxyl Radicals and Trapped Holes in Photocatalysis. A Pulse Radiolysis Study. J. Phys. Chem. 1991, 95, 5166–5170. [Google Scholar] [CrossRef]
- Tryba, B.; Toyoda, M.; Morawski, A.W.; Nonaka, R.; Inagaki, M. Photocatalytic Activity and OH Radical Formation on TiO2 in the Relation to Crystallinity. Appl. Catal. B Environ. 2007, 71, 163–168. [Google Scholar] [CrossRef]
- Nosaka, Y.; Komori, S.; Yawata, K.; Hirakawa, T.; Nosaka, A.Y. Photocatalytic OH Radical Formation in TiO2 Aqueous Suspension Studied by Several Detection Methods. Phys. Chem. Chem. Phys. 2003, 5, 4731–4735. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Tang, L.; Xin, K.; Bai, A.; Yu, Y. Synthesis, Photocatalytic Activity, and Photogenerated Hydroxyl Radicals of Monodisperse Colloidal ZnO Nanospheres. Appl. Surf. Sci. 2015, 357, 1928–1938. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nosaka, Y. Properties of O2•- and OH• Formed in TiO2 Aqueous Suspensions by Photocatalytic Reaction and the Influence of H2O2 and Some Ions. Langmuir 2002, 18, 3247–3254. [Google Scholar] [CrossRef]
- Kawamoto, K.; Tsujimoto, Y. Effects of the Hydroxyl Radical and Hydrogen Peroxide on Tooth Bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.R.; Tromp, J.; Hair, M.L. Photogeneration of Hydrogen Peroxide in Aqueous TiO2 Dispersions. Can. J. Chem. 1985, 63, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Bahnemann, D.W.; Hoffmann, M.R.; Hong, A.P.; Kormann, C. Photocatalytic Formation of Hydrogen Peroxide. ACS Publ. 1987, 349, 120–132. [Google Scholar]
- Baur, E.; Neuweiler, C. Photolytic Formation of Hydrogen Peroxide. Helv. Chim. Acta 1927, 10, 901–907. [Google Scholar] [CrossRef]
- Calvert, J.G.; Theurer, K.; Rankin, G.T.; MacNevin, W.M. A Study of the Mechanism of the Photochemical Synthesis of Hydrogen Peroxide at Zinc Oxide Surfaces1. J. Am. Chem. Soc. 1954, 76, 2575–2578. [Google Scholar] [CrossRef]
- Munuera, G.; González-Elipe, A.R.; Soria, J.; Sanz, J. Photo-Adsorption and Photo-Desorption of Oxygen on Highly Hydroxylated TiO2 Surfaces. Part 3.—Role of H2O2 in Photo-Desorption of O2. J. Chem. Soc. 1980, 76, 1535–1546. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic Production of H2O2 and Organic Peroxides in Aqueous Suspensions of TiO2, ZnO, and Desert Sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, C.; Zhao, J. Mechanism of Photodecomposition of H2O2 on TiO2 Surfaces under Visible Light Irradiation. Langmuir 2001, 17, 4118–4122. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Sahel, K.; Elsellami, L.; Mirali, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen Peroxide and Photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic Reactivity for O2− and OH Radical Formation in Anatase and Rutile TiO2 Suspension as the Effect of H2O2 Addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Jang, E.S.; Won, J.-H.; Hwang, S.-J.; Choy, J.-H. Fine Tuning of the Face Orientation of ZnO Crystals to Optimize Their Photocatalytic Activity. Adv. Mater. 2006, 18, 3309–3312. [Google Scholar] [CrossRef]
- Domènech, X.; Ayllón, J.A.; Peral, J. H2O2 Formation from Photocatalytic Processes at the ZnO/Water Interface. Environ. Sci. Pollut. Res. 2001, 8, 285–287. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Carraway, E.R.; Hoffmann, M.R. Photocatalytic Production of H2O2 and Organic Peroxides on Quantum-Sized Semiconductor Colloids. Environ. Sci. Technol. 1994, 28, 776–785. [Google Scholar] [CrossRef]
- Mrowetz, M.; Selli, E. Photocatalytic Degradation of Formic and Benzoic Acids and Hydrogen Peroxide Evolution in TiO2 and ZnO Water Suspensions. J. Photochem. Photobiol. A Chem. 2006, 180, 15–22. [Google Scholar] [CrossRef]
- Pichat, P.; Guillard, C.; Amalric, L.; Renard, A.-C.; Plaidy, O. Assessment of the Importance of the Role of H2O2 and O2o− in the Photocatalytic Degradation of 1,2-Dimethoxybenzene. Sol. Energy Mater. Sol. Cells 1995, 38, 391–399. [Google Scholar] [CrossRef]
- Khodja, A.A.; Sehili, T.; Pilichowski, J.-F.; Boule, P. Photocatalytic Degradation of 2-Phenylphenol on TiO2 and ZnO in Aqueous Suspensions. J. Photochem. Photobiol. A Chem. 2001, 141, 231–239. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Poulios, I.; Micropoulou, E.; Panou, R.; Kostopoulou, E. Photooxidation of Eosin Y in the Presence of Semiconducting Oxides. Appl. Catal. B Environ. 2003, 41, 345–355. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.-M. Effect of Hydrogen Peroxide on the Destruction of Organic Contaminants-Synergism and Inhibition in a Continuous-Mode Photocatalytic Reactor. Appl. Catal. B Environ. 2004, 50, 259–269. [Google Scholar] [CrossRef]
- Barakat, M.A.; Tseng, J.M.; Huang, C.P. Hydrogen Peroxide-Assisted Photocatalytic Oxidation of Phenolic Compounds. Appl. Catal. B Environ. 2005, 59, 99–104. [Google Scholar] [CrossRef]
- Domingues, F.S.; Freitas, T.K.F.d.S.; de Almeida, C.A.; de Souza, R.P.; Ambrosio, E.; Palácio, S.M.; Garcia, J.C. Hydrogen Peroxide-Assisted Photocatalytic Degradation of Textile Wastewater Using Titanium Dioxide and Zinc Oxide. Environ. Technol. 2019, 40, 1223–1232. [Google Scholar] [CrossRef]
- Jeffery, G.A. Elements of X-ray Diffraction (Cullity, B.D.). J. Chem. Educ. 1957, 34, A178. [Google Scholar] [CrossRef] [Green Version]
- Setaka, M.; Sancier, K.M.; Kwan, T. Electron Spin Resonance Investigation of Electrical Conductivity Parameters of Zinc Oxide during Surface Reactions. J. Catal. 1970, 16, 44–52. [Google Scholar] [CrossRef]
- Schneider, J.J.; Hoffmann, R.C.; Engstler, J.; Dilfer, S.; Klyszcz, A.; Erdem, E.; Jakes, P.; Eichel, R.A. Zinc Oxide Derived from Single Source Precursor Chemistry under Chimie Douce Conditions: Formation Pathway, Defect Chemistry and Possible Applications in Thin Film Printing. J. Mater. Chem. 2009, 19, 1449. [Google Scholar] [CrossRef]
- Hoffmann, K.; Hahn, D. Electron Spin Resonance of Lattice Defects in Zinc Oxide. Phys. Stat. Sol. A 1974, 24, 637–648. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native Point Defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Danilenko, I.; Gorban, O.; Maksimchuk, P.; Viagin, O.; Malyukin, Y.; Gorban, S.; Volkova, G.; Glasunova, V.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; et al. Photocatalytic Activity of ZnO Nanopowders: The Role of Production Techniques in the Formation of Structural Defects. Catal. Today 2019, 328, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Xuan Sang, N.; Minh Quan, N.; Huu Tho, N.; Tri Tuan, N.; Thanh Tung, T. Mechanism of Enhanced Photocatalytic Activity of Cr-Doped ZnO Nanoparticles Revealed by Photoluminescence Emission and Electron Spin Resonance. Semicond. Sci. Technol. 2019, 34, 025013. [Google Scholar] [CrossRef]
- Kasai, P.H. Electron Spin Resonance Studies of Donors and Acceptors in ZnO. Phys. Rev. 1963, 130, 989–995. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Liu, J.; Rojas-Andrade, M.D.; Chata, G.; Peng, Y.; Roseman, G.; Lu, J.-E.; Millhauser, G.L.; Saltikov, C.; Chen, S. Photo-Enhanced Antibacterial Activity of ZnO/Graphene Quantum Dot Nanocomposites. Nanoscale 2018, 10, 158–166. [Google Scholar] [CrossRef]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; De Stefano, L.; Aronne, A.; Vaiano, V. F-Doped ZnO Nano-and Meso-Crystals with Enhanced Photocatalytic Activity in Diclofenac Degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef]
- Cerrato, E.; Paganini, M.C.; Giamello, E. Photoactivity under Visible Light of Defective ZnO Investigated by EPR Spectroscopy and Photoluminescence. J. Photochem. Photobiol. A Chem. 2020, 397, 112531. [Google Scholar] [CrossRef]
- Pinteala, M.; Schlick, S. Direct ESR Detection and Spin Trapping of Radicals Generated by Reaction of Oxygen Radicals with Sulfonated Poly (Ether Ether Ketone)(SPEEK) Membranes. Polym. Degrad. Stab. 2009, 94, 1779–1787. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin Trapping: ESR Parameters of Spin Adducts 1474 1528V. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Augugliaro, V.; Palmisano, L.; Sclafani, A.; Minero, C.; Pelizzetti, E. Photocatalytic Degradation of Phenol in Aqueous Titanium Dioxide Dispersions. Toxicol. Environ. Chem. 1988, 16, 89–109. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Tanaka, M.; Itaya, A. Heterogeneous Photocatalytic Decomposition of Phenol over TiO2 Powder. Bull. Chem. Soc. Jpn. 1985, 58, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, Y.; Xu, J.; Bai, X.; Zong, R.; Zhu, Y. Degradation and Mineralization Mechanism of Phenol by BiPO4 Photocatalysis Assisted with H2O2. Appl. Catal. B Environ. 2013, 142–143, 561–567. [Google Scholar] [CrossRef]
- Turki, A.; Guillard, C.; Dappozze, F.; Ksibi, Z.; Berhault, G.; Kochkar, H. Phenol Photocatalytic Degradation over Anisotropic TiO2 Nanomaterials: Kinetic Study, Adsorption Isotherms and Formal Mechanisms. Appl. Catal. B Environ. 2015, 163, 404–414. [Google Scholar] [CrossRef]
- Mediouni, N.; Guillard, C.; Dappozze, F.; Khrouz, L.; Parola, S.; Colbeau-Justin, C.; Amara, A.B.H.; Rhaiem, H.B.; Jaffrezic-Renault, N.; Namour, P. Impact of Structural Defects on the Photocatalytic Properties of ZnO. J. Hazard. Mater. Adv. 2022, 6, 100081. [Google Scholar] [CrossRef]











| ZnA4T | ZnH4T | ZnP4T | ZnO-Com | |
|---|---|---|---|---|
| Crystallite size (nm) | 19 | 23 | 14 | 40 |
| BET (m2/g) | 34 | 17 | 17 | 17 |
| C°OH (mM) | 11.2 | 8.2 | 6.1 | 11.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mediouni, N.; Dappozze, F.; Khrouz, L.; Parola, S.; Amara, A.B.H.; Rhaiem, H.B.; Jaffrezic-Renault, N.; Namour, P.; Guillard, C. Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase. Catalysts 2022, 12, 1445. https://doi.org/10.3390/catal12111445
Mediouni N, Dappozze F, Khrouz L, Parola S, Amara ABH, Rhaiem HB, Jaffrezic-Renault N, Namour P, Guillard C. Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase. Catalysts. 2022; 12(11):1445. https://doi.org/10.3390/catal12111445
Chicago/Turabian StyleMediouni, Nouha, Frederic Dappozze, Lhoussain Khrouz, Stephane Parola, Abdesslem Ben Haj Amara, Hafsia Ben Rhaiem, Nicole Jaffrezic-Renault, Philippe Namour, and Chantal Guillard. 2022. "Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase" Catalysts 12, no. 11: 1445. https://doi.org/10.3390/catal12111445