Biocatalysts in Synthesis of Microbial Polysaccharides: Properties and Development Trends
Abstract
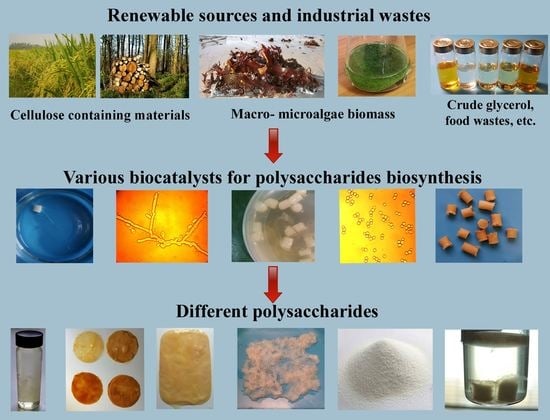
:1. Introduction
2. Immobilized Biocatalysts for the Synthesis of Exopolysaccharides
3. New Natural, Mutated and Genetically Modified Strains as BTCs for PS Synthesis
4. BTCs as Native or Artificial (Co-Cultured) Consortia for EPS Production
5. Features of BTCs Determine the Palette of Substrates (Types of Biomass and Methods of Its Pretreatment) for EPS Synthesis
6. Factors Affecting the Properties of BTCs Used for PS Synthesis
7. Preparation of Nanocomposites in the Process of Biocatalytic PS Synthesis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradali, M.F.; Rehm, B.H. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Aanisah, N.; Wardhana, Y.W.; Chaerunisaa, A.Y.; Budiman, A. Review on modification of glucomannan as an excipient in solid dosage forms. Polymers 2022, 14, 2550. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound healing composite materials of bacterial cellulose and zinc oxide nanoparticles with immobilized betulin diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, M.; Balas, C. Bacterial polysaccharides versatile medical uses. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J.M., Randhouani, H., Reis, R.L., Eds.; Springer Cham: Cham, Switzerland, 2022; pp. 859–891. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sen, I.K.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agric. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Atykyan, N.; Revin, V.; Shutova, V. Raman and FT-IR Spectroscopy investigation the cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Express 2020, 10, 84. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Schmid, J. Recent insights in microbial exopolysaccharide biosynthesis and engineering strategies. Curr. Opin. Biotechnol. 2018, 53, 130–136. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Jin, J.; Murad, M.S.; Mu, G.; Wu, X. Comparison on properties between normal and A2 bovine milk fermented using commercial bacteria mixed with/without two probiotics from human milk. Int. J. Biol. Macromol 2022, 216, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Meng, K.; Yang, X.; Liu, J.; Yu, J.; Zheng, C.; Cao, W.; Liu, H. Identification of a quorum sensing system regulating capsule polysaccharide production and biofilm formation in Streptococcus zooepidemicus. Front. Cell. Infect. Microbiol. 2019, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Chen, N.; Li, J.; Han, C.F.; Wang, S.; Hao, L.M.; Jia, S.R.; Han, P.P. The effects of quorum sensing molecule farnesol on the yield and activity of extracellular polysaccharide from Grifola frondosa in liquid fermentation. Int. J. Biol. Macromol. 2021, 191, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.V.; Efremenko, E.N. Highly concentrated populations of Aureobasidium pullulans cells in biocatalytic pullulan production processes. Catal. Ind. 2017, 9, 344–348. [Google Scholar] [CrossRef]
- Zur, J.; Wojcieszynska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Exopolysaccharide production during batch cultures with free and immobilized Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2003, 95, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Growth and exopolysaccharide production during free and immobilized cell chemostat culture of Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2005, 98, 272–284. [Google Scholar] [CrossRef]
- EL-Gizawy, S.A.; Barakat, O.S.; Sharaf, O.M.; EL-Shafei, K.; Fathy, F.A.; EL-Sayed, H.S. Effect of growth conditions on the production of exopolysaccharides by microencapsulated Lactobacillus bulgaricus and use it to improve quality of Kareish cheese. J. Appl. Sci. Res. 2013, 9, 1097–1109. [Google Scholar]
- Adebayo-Tayo, B.; Agidigbi, O.; Alao, S. Comparative influence of immobilization medium and mutation on EPS-production by L. plantarum MK 02 isolated from fermented milk. Trakia J. Sci. 2017, 1, 30. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Exopolysaccharide production and prevention of syneresis in starch using encapsulated probiotic Lactobacillus plantarum. Food Technol. Biotechnol. 2010, 48, 484–489. [Google Scholar]
- Efremenko, E.N. Immobilized Cells: Biocatalysts and Processes; RIOR: Moscow, Russia, 2018; p. 409. [Google Scholar] [CrossRef]
- Nugroho, D.A.; Aji, P. Characterization of nata de coco produced by fermentation of immobilized Acetobacter xylinum. Agric. Agric. Sci. Procedia 2015, 3, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Matyash, M.; Despang, F.; Ikonomidou, C.; Gelinsky, M. Swelling and mechanical properties of alginate hydrogels with respect to promotion of neural growth. Tissue Eng. Part C Methods 2014, 20, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and struggle of opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341. [Google Scholar] [CrossRef]
- Senko, O.; Gladchenko, M.; Maslova, O.; Efremenko, E. Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas. Catalysts 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. Immobilised cells of Pachysolen tannophilus yeast for ethanol production from crude glycerol. New Biotechnol. 2017, 34, 54–58. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as biocatalyst for intensive bacterial cellulose production from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Yáñez-Fernández, J.; Castro-Muñoz, R. Microfiltration-mediated extraction of dextran produced by Leuconostoc mesenteroides SF3. Food Bioprod. Process. 2020, 119, 317–328. [Google Scholar] [CrossRef]
- Diana, C.R.; Humberto, H.S.; Jorge, Y.F. Structural characterization and rheological properties of dextran produced by native strains isolated of Agave salmiana. Food Hydrocoll. 2019, 90, 1–8. [Google Scholar] [CrossRef]
- Netsopa, S.; Niamsanit, S.; Sakloetsakun, D.; Milintawisamai, N. Characterization and rheological behavior of dextran from Weissella confusa R003. Int. J. Polym. Sci. 2018, 2018, 5790526. [Google Scholar] [CrossRef] [Green Version]
- Ma’unatin, A.; Harijono, H.; Zubaidah, E.; Rifa’i, M. Dextran production using Leuconostoc mesenteroides strains isolated from Borassus flabellifer sap. Biodivers. J. Biol. Divers. 2022, 23, 1154–1158. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Wang, Y.; Wu, H.; Fang, Y.; Ma, J.; Jiang, M. Production and characterization of insoluble α-1, 3-linked glucan and soluble α-1, 6-linked dextran from Leuconostoc pseudomesenteroides G29. Chin. J. Chem. Eng. 2021, 39, 211–218. [Google Scholar] [CrossRef]
- Yu, Y.J.; Chen, Z.; Chen, P.T.; Ng, I.S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Coda, R.; Maina, N.H.; Granchi, L. Isolation and characterization of indigenous Weissella confusa for in situ bacterial exopolysaccharides (EPS) production in chickpea sourdough. Food Res. Int. 2020, 138, 109785. [Google Scholar] [CrossRef]
- Esmaeilnejad-Moghadam, B.; Mokarram, R.R.; Hejazi, M.A.; Khiabani, M.S.; Keivaninahr, F. Low molecular weight dextran production by Leuconostoc mesenteroides strains: Optimization of a new culture medium and the rheological assessments. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100181. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Venkatachalam, P.; Karuppiah, S. Cost-effective production of dextran using Saccharum officinarum juice (SOJ) as a potential feedstock: Downstream processing and characterization. Biomass Convers. Biorefin. 2022, 12, 4863–4875. [Google Scholar] [CrossRef]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ spent grain as substrate for dextran biosynthesis by Leuconostoc pseudomesenteroides DSM20193 and Weissella confusa A16. Microb. Cell Fact. 2021, 20, 23. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Puertas, A.I.; Prieto, A.; López, P.; Cobos, M.; Miranda, J.I.; Marieta, C.; Ruas-Madiedo, P.; Dueñas, M.T. Characterization of dextrans produced by Lactobacillus mali CUPV271 and Leuconostoc carnosum CUPV411. Food Hydrocoll. 2019, 89, 613–622. [Google Scholar] [CrossRef]
- Hu, Y.; Gänzle, M.G. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10 M. Int. J. Food Microbiol. 2018, 280, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.N.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A. Structural analysis and characterization of dextran produced by wild and mutant strains of Leuconostoc mesenteroides. Carbohydr. Polym. 2014, 99, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, N.A.; Senko, O.V.; Efremenko, E.N. Biocatalytic production of extracellular exopolysaccharide dextran synthesized by cells of Leuconostoc mesenteroides. Catal. Ind. 2017, 9, 339–343. [Google Scholar] [CrossRef]
- Qader, S.A.U.; Aman, A. Low molecular weight dextran: Immobilization of cells of Leuconostoc mesenteroides KIBGE HA1 on calcium alginate beads. Carbohydr. Polym. 2012, 87, 2589–2592. [Google Scholar] [CrossRef]
- Veerapandian, B.; Shanmugam, S.R.; Varadhan, S.; Sarwareddy, K.K.; Mani, K.P.; Ponnusami, V. Levan production from sucrose using chicken feather peptone as a low cost supplemental nutrient source. Carbohydr. Polym. 2020, 227, 115361. [Google Scholar] [CrossRef]
- Taran, M.; Lotfi, M.; Safaei, M. Optimal conditions for levan biopolymer production and its use in the synthesis of bactericidal levan-zno nanocomposite. Biotechnologia 2019, 100, 397–405. [Google Scholar] [CrossRef]
- Ramya, P.; Sangeetha, D.; Anooj, E.S.; Gangadhar, L. Studies on the production and optimization of levan from Bacillus sp. Ann. Trop. Med. Public Health 2020, 23, 1188–1197. [Google Scholar] [CrossRef]
- Moussa, T.A.; Al-Qaysi, S.A.; Thabit, Z.A.; Kadhem, S.B. Microbial levan from Brachybacterium phenoliresistens: Characterization and enhancement of production. Process Biochem. 2017, 57, 9–15. [Google Scholar] [CrossRef]
- Lorenzetti, M.F.S.; Moro, M.R.; García-Cruz, C.H. Alginate/PVA beads for levan production by Zymomonas mobilis. J. Food Process Eng. 2015, 38, 31–36. [Google Scholar] [CrossRef]
- Santos, V.A.Q.; Cruz, C.H.G. Ethanol and levan production by sequential bath using Zymomonas mobilis immobilized on alginate and chitosan beads. Acta Sci.–Technol. 2016, 38, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Santos, V.A.Q.; Cruz, C.H.G. Zymomonas mobilis immobilized on loofa sponge and sugarcane bagasse for levan and ethanol production using repeated batch fermentation. Braz. J. Chem. Eng. 2017, 34, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, A.; Akyliyaevna, K.A.; Zaman, W.Q.; Hussain, M.H.; Mohsin, M.Z.; Al-Rashed, S.; Tan, X.; Tian, X.; Aida, K.; Tariq, M.; et al. Kinetically modelled approach of xanthan production using different carbon sources: A study on molecular weight and rheological properties of xanthan. Int. J. Biol. Macromol. 2021, 193, 1226–1236. [Google Scholar] [CrossRef]
- Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Dodić, J. Utilisation of winery wastewater for xanthan production in stirred tank bioreactor: Bioprocess modelling and optimisation. Food Bioprod. Process. 2019, 117, 113–125. [Google Scholar] [CrossRef]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Gao, M.J.; Zhu, L.; Zhan, X.B. High production of xanthan gum by a glycerol-tolerant strain Xanthomonas campestris WXLB-006. Prep. Biochem. Biotechnol. 2017, 47, 468–472. [Google Scholar] [CrossRef]
- Mesquita, R.A.; Hassemer, G.; Marchiori, V.; Kiedis, J.; Valduga, E.; Junges, A.; Malvessi, E.; Cansian, R.L.; Zeni, J. Synthesis of xanthan gum from Xanthomonas campestris immobilized in polyurethane. Ind. Biotechnol. 2018, 14, 276–281. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Razmjooei, M.; Safdarianghomsheh, R.; Shad, E.; Delvigne, F.; Khalesi, M. Semi-continuous production of xanthan in biofilm reactor using Xanthomonas campestris. J. Biotechnol. 2021, 328, 1–11. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D. Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int. J. Biol. Macromol. 2016, 82, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Nejadmansouri, M.; Shad, E.; Razmjooei, M.; Safdarianghomsheh, R.; Delvigne, F.; Khalesi, M. Production of xanthan gum using immobilized Xanthomonas campestris cells: Effects of support type. Biochem. Eng. J. 2020, 157, 107554. [Google Scholar] [CrossRef]
- Meena, S.; Tripathi, A.D.; Ts, R.L. Optimization and characterization of Alginic acid synthesized from a novel strain of Pseudomonas stutzeri. Biotechnol. Rep. 2020, 27, e00517. [Google Scholar] [CrossRef]
- Dudun, A.A.; Akoulina, E.A.; Zhuikov, V.A.; Makhina, T.K.; Voinova, V.V.; Belishev, N.V.; Khaydapova, D.D.; Shaitan, K.V.; Bonartseva, G.A.; Bonartsev, A.P. Competitive biosynthesis of bacterial alginate using Azotobacter vinelandii 12 for tissue engineering applications. Polymers 2022, 14, 131. [Google Scholar] [CrossRef]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical optimization of cultural parameters for the optimized production of alginic acid using apple (Malus domestica) peels through solid-state fermentation. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- García, A.; Castillo, T.; Ramos, D.; Ahumada-Manuel, C.L.; Núñez, C.; Galindo, E.; Büchs, J.; Peña, C. Molecular weight and viscosifying power of alginates produced by mutant strains of Azotobacter vinelandii under microaerophilic conditions. Biotechnol. Rep. 2020, 26, e00436. [Google Scholar] [CrossRef]
- Jiang, H.; Xue, S.J.; Li, Y.F.; Liu, G.L.; Chi, Z.M.; Hu, Z.; Chi, Z. Efficient transformation of sucrose into high pullulan concentrations by Aureobasidium melanogenum TN1-2 isolated from a natural honey. Food Chem. 2018, 257, 29–35. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N. Biochemical and molecular characterization of a new pullulan producer Rhodosporidium paludigenum PUPY-06. J. Appl. Biol. Biotechnol. 2018, 6, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhu, Y.; Zhang, G.; Liu, H.; Wei, Y.; Wang, P.; Xian, M.; Xiang, H.; Zhang, H. Optimization and characterization of pullulan production by a newly isolated high-yielding strain Aureobasidium melanogenum. Prep. Biochem. Biotechnol. 2019, 49, 557–566. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr. Polym. 2020, 229, 115513. [Google Scholar] [CrossRef]
- Greser, A.B.; Avcioglu, N.H. Optimization and physicochemical characterization of bacterial cellulose by Komagataeibacter nataicola and Komagataeibacter maltaceti strains isolated from grape, thorn apple and apple vinegars. Arch. Microbiol. 2022, 204, 465. [Google Scholar] [CrossRef] [PubMed]
- Leonarski, E.; Cesca, K.; Pinto, C.C.; González, S.Y.; de Oliveira, D.; Poletto, P. Bacterial cellulose production from acerola industrial waste using isolated kombucha strain. Cellulose 2022, 29, 7613–7627. [Google Scholar] [CrossRef]
- Skiba, E.A.; Gladysheva, E.K.; Golubev, D.S.; Budaeva, V.V.; Aleshina, L.A.; Sakovich, G.V. Self-standardization of quality of bacterial cellulose produced by Medusomyces gisevii in nutrient media derived from Miscanthus biomass. Carbohydr. Polym. 2021, 252, 117178. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2018, 102, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.S.; Harrison, M.D.; Speight, R.E.; O’Hara, I.M.; Zhang, Z. Efficient production of fructo-oligosaccharides from sucrose and molasses by a novel Aureobasidium pullulan strain. Biochem. Eng. J. 2020, 163, 107747. [Google Scholar] [CrossRef]
- Castro, C.C.; Nobre, C.; Duprez, M.E.; De Weireld, G.; Hantson, A.L. Screening and selection of potential carriers to immobilize Aureobasidium pullulans cells for fructo-oligosaccharides production. Biochem. Eng. J. 2017, 118, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Rajeswari, K.; Divya, P.; Ferlin, M.; Rajeshwari, C.T.; Vanavil, B. Optimization and production of curdlan gum using Bacillus cereus pr3 isolated from rhizosphere of leguminous plant. Prep. Biochem. Biotechnol. 2018, 48, 408–418. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; Arafat, H.H.; Elsehemy, I.A.; Basha, M. Optimization, purification and physicochemical characterization of curdlan produced by Paenibacillus sp. strain NBR-10. Biosci. Biotechnol. Res. Asia 2016, 13, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Anane, R.F.; Sun, H.; Zhao, L.; Wang, L.; Lin, C.; Mao, Z. Improved curdlan production with discarded bottom parts of Asparagus spear. Microb. Cell Fact. 2017, 16, 59. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Shao, Z.; Jiang, D.; Gao, H.; Yang, X. Curdlan production from cassava starch hydrolysates by Agrobacterium sp. DH-2. Bioprocess Biosyst. Eng. 2022, 45, 969–979. [Google Scholar] [CrossRef]
- Gao, H.; Xie, F.; Zhang, W.; Tian, J.; Zou, C.; Jia, C.; Jin, M.; Huang, J.; Chang, Z.; Yang, X.; et al. Characterization and improvement of curdlan produced by a high-yield mutant of Agrobacterium sp. ATCC 31749 based on whole-genome analysis. Carbohydr. Polym. 2020, 245, 116486. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Martinez, C.; Pereira Ruiz, S.; Carvalho Fenelon, V.; Rodrigues de Morais, G.; Luciano Baesso, M.; Matioli, G. Characterization of curdlan produced by Agrobacterium sp. IFO 13140 cells immobilized in a loofa sponge matrix, and application of this biopolymer in the development of functional yogurt. J. Sci. Food Agric. 2016, 96, 2410–2417. [Google Scholar] [CrossRef]
- Pedroso, G.B.; Silva, L.O.; Araujo, R.B.; Saldanha, L.F.; Denardi, L.; Martins, A.F. An innovative approach for the biotechnological production of succinoglycan from rice husks. Ind. Crops Prod. 2019, 137, 615–627. [Google Scholar] [CrossRef]
- de Oliveira Delani, T.C.; Sampaio, A.R.; da Silva Palácios, R.; Sato, F.; Ruiz, S.P.; de Oliveira Petkowicz, C.L.; Reichembach, L.H.; Miyoshi, J.H.; Nascimento, M.G.; Matioli, G. Rheological and structural characterization of succinoglycan obtained by bioconversion of the agroindustrial residue deproteinized whey. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Gao, H.; Yang, L.; Tian, J.; Huang, L.; Huang, D.; Zhang, W.; Xie, F.; Niu, Y.; Jin, M.; Jia, C.; et al. Characterization and rheological properties analysis of the succinoglycan produced by a high-yield mutant of Rhizobium radiobacter ATCC 19358. Int. J. Biol. Macromol. 2021, 166, 61–70. [Google Scholar] [CrossRef]
- Ruiz, S.P.; Martinez, C.O.; Noce, A.S.; Sampaio, A.R.; Baesso, M.L.; Matioli, G. Biosynthesis of succinoglycan by Agrobacterium radiobacter NBRC 12665 immobilized on loofa sponge and cultivated in sugar cane molasses. Structural and rheological characterization of biopolymer. J. Mol. Catal. B Enzym. 2015, 122, 15–28. [Google Scholar] [CrossRef]
- Meng, X.; Dobruchowska, J.M.; Pijning, T.; Gerwig, G.J.; Dijkhuizen, L. Synthesis of new hyperbranched α-glucans from sucrose by Lactobacillus reuteri 180 glucansucrase mutants. J. Agric. Food Chem. 2016, 64, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Avcioglu, N.H.; Birben, M.; Bilkay, I.S. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new Kombucha consortium. Process Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Betlej, I.; Boruszewski, P.; Dubis, D.; Wilkowski, J.; Krajewski, K.J.; Zawadzki, J. Influence of SCOBY microorganisms’ cultivation conditions on the synthesis efficiency and selected qualities of bacterial cellulose. BioResources 2021, 16, 6147–6158. [Google Scholar] [CrossRef]
- Tapias, Y.A.R.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by Kombucha symbiotic community cultured on different herbal infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, L.; Ding, H.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Enhanced production of curdlan by coupled fermentation system of Agrobacterium sp. ATCC 31749 and Trichoderma harzianum GIM 3.442. Carbohydr. Polym. 2017, 157, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Senko, O.; Akopyan, A.; Lysenko, S.; Anisimov, A.; Efremenko, E. Nanocatalysts for oxidative desulfurization of liquid fuel: Modern solutions and the perspectives of application in hybrid chemical-biocatalytic processes. Catalysts 2021, 11, 1131. [Google Scholar] [CrossRef]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013, 12, 93. [Google Scholar] [CrossRef] [Green Version]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E. Enzymatically functionalized composite materials based on nanocellulose and poly(vinyl alcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.M.; Kadoguchi, E.; Segato, F.; da Silva, S.S.; Chandel, A.K. Production of cellulases by Aureobasidium pullulans LB83: Optimization, characterization, and hydrolytic potential for the production of cellulosic sugars. Prep. Biochem. Biotechnol. 2021, 51, 153–163. [Google Scholar] [CrossRef]
- Mulay, Y.R.; Deopurkar, R.L. Production of amylase from indigenously isolated strain of Aureobasidium pullulans and its hyper production mutant. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 287–293. [Google Scholar] [CrossRef]
- Shi, Q.S.; Feng, J.; Li, W.R.; Zhou, G.; Chen, A.M.; Ouyang, Y.S.; Chen, Y.B. Effect of different conditions on the average degree of polymerization of bacterial cellulose produced by Gluconacetobacter intermedius BC-41. Cellul. Chem. Technol. 2013, 47, 503–508. [Google Scholar]
- Al-Shamary, E.E.; Al-Darwash, A.K. Influence of fermentation condition and alkali treatment on the porosity and thickness of bacterial cellulose membranes. TOJSAT 2013, 3, 194–203. [Google Scholar]
- Keshk, S.M.A.S. Bacterial cellulose production and its industrial applications. J. Bioprocess Biotech. 2014, 4, 150. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.; Nair, G.R.; Lay, M.C.; Swan, J.E.; Umar, R. Glycerol as a cheaper carbon source in bacterial cellulose (BC) production by Gluconobacter xylinus DSM46604 in batch fermentation system. MJAS 2015, 19, 1131–1136. [Google Scholar]
- Suwanposri, A.; Yukphan, P.; Yamada, Y.; Ochaikul, D. Statistical optimisation of culture conditions for biocellulose production by Komagataeibacter sp. PAP1 using soya bean whey. Maejo Int. J. Sci. Technol. 2014, 8, 1. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.A.; Xiong, L.; Guo, H.-J.; Luo, J.; Wang, B.; Zhang, H.-R.; Lin, X.-Q.; Chen, X.-D. Utilization of corncob acid hydrolysate for bacterial cellulose production by Gluconacetobacter xylinus. Appl. Biochem. Biotechnol. 2015, 175, 1678–1688. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, X. Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl. Biochem. Biotechnol. 2014, 172, 3844–3861. [Google Scholar] [CrossRef]
- Ul-Islam1, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Kashcheyeva, E.I.; Gismatulina, Y.A.; Mironova, G.F.; Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Zolotuhin, V.N.; Shavyrkina, N.A.; Kortusov, A.N.; Korchagina, A.A. Properties and hydrolysis behavior of celluloses of different origin. Polymers 2022, 14, 3899. [Google Scholar] [CrossRef]
- Morgan, J.L.W.; McNamara, J.T.; Zimmer, J. Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat. Struct. Mol. Biol. 2014, 21, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Lopes, B.D.; Lessa, V.L.; Silva, B.M.; Carvalho, M.A.D.; Schnitzler, E.; Lacerda, L.G. Xanthan gum: Properties, production conditions, quality and economic perspective. J. Food Nutr. Res. 2015, 54, 185–194. [Google Scholar]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Zhan, X.B.; Lin, C.C.; Zhang, H.T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2012, 93, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyari, M.; Moosavi-Nasab, M.; Askari, H. Optimization of succinoglycan hydrocolloid production by Agrobacterium radiobacter grown in sugar beet molasses and investigation of its physicochemical characteristics. Food Hydrocoll. 2015, 45, 18–29. [Google Scholar] [CrossRef]
- Ribeiro, V.A.; Burkert, C.A.V. Exopolysaccharides produced by Rhizobium: Production, composition and rheological properties. J. Polym. Biopolym. Phys. Chem. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Hu, H.; Catchmark, J.M.; Demirci, A. Co-culture fermentation on the production of bacterial cellulose nanocomposite produced by Komagataeibacter hansenii. Carbohydr. Polym. Technol. Appl. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Lyagin, I.; Maslova, O.; Stepanov, N.; Presnov, D.; Efremenko, E. Assessment of composite with fibers as a support for antibacterial nanomaterials: A case study of bacterial cellulose, polylactide and usual textile. Fibers 2022, 10, 70. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef]
- Kianpour, S.; Ebrahiminezhad, A.; Mohkam, M.; Tamaddon, A.M.; Dehshahri, A.; Heidari, R.; Ghasemi, Y. Physicochemical and biological characteristics of the nanostructured polysaccharide-iron hydrogel produced by microorganism Klebsiella oxytoca. J. Basic Microbiol. 2017, 57, 132–140. [Google Scholar] [CrossRef]
- Gabryś, T.; Fryczkowska, B.; Fabia, J.; Biniaś, D. Preparation of an active dressing by in situ biosynthesis of a bacterial cellulose–graphene oxide composite. Polymers 2022, 14, 2864. [Google Scholar] [CrossRef]
- Dhar, P.; Pratto, B.; Cruz, A.J.G.; Bankar, S. Valorization of sugarcane straw to produce highly conductive bacterial cellulose/graphene nanocomposite films through in situ fermentation: Kinetic analysis and property evaluation. J. Clean. Prod. 2019, 238, 117859. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Jo, I.; Cho, S.P.; Sung, D.; Ryu, S.; Park, M.; Min, K.A.; Kim, J.; Hong, S.; et al. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials 2015, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhong, C.; Zheng, X.; Ye, L.; Wan, T.; Jia, S.R. Oriented bacterial cellulose-glass fiber nanocomposites with enhanced tensile strength through electric field. Fibers Polym. 2017, 18, 1408–1412. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In situ synthesized porous bacterial cellulose/poly (vinyl alcohol)-based silk sericin and azithromycin release system for treating chronic wound biofilm. Macromol. Biosci. 2022, 22, 2200201. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Catchmark, J.M.; Demirci, A. Effects of pullulan additive and co-culture of Aureobasidium pullulans on bacterial cellulose produced by Komagataeibacter hansenii. Bioprocess Biosyst. Eng. 2022, 45, 573–587. [Google Scholar] [CrossRef]
- Liu, K.; Catchmark, J.M. Enhanced mechanical properties of bacterial cellulose nanocomposites produced by co-culturing Gluconacetobacter hansenii and Escherichia coli under static conditions. Carbohydr. Polym. 2019, 219, 12–20. [Google Scholar] [CrossRef]
- Zhantlessova, S.; Savitskaya, I.; Kistaubayeva, A.; Ignatova, L.; Talipova, A.; Pogrebnjak, A.; Digel, I. Advanced “green” prebiotic composite of bacterial cellulose/pullulan based on synthetic biology-powered microbial coculture strategy. Polymers 2022, 14, 3224. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Tailoring the in situ conformation of bacterial cellulose-graphene oxide spherical nanocarriers. Int. J. Biol. Macromol. 2020, 163, 1249–1260. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Yao, F.; Yang, Z.; Li, W.; Wang, J.; Xu, X.; Hu, J.; Wan, Y. Layer-by-layer assembled bacterial cellulose/graphene oxide hydrogels with extremely enhanced mechanical properties. Nano-Micro Lett. 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Tang, J.; Henzie, J.; Li, Y.; Xu, X.; Chen, T.; Wang, J.; Ide, Y.; Bando, Y.; Yamauchi, Y. Assembly of hollow carbon nanospheres on graphene nanosheets and creation of iron–nitrogen-doped porous carbon for oxygen reduction. ACS Nano 2018, 12, 5674–5683. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Cybulska, J.; Kozioł, A.; Pieczywek, P.M.; Zdunek, A. Simultaneous influence of pectin and xyloglucan on structure and mechanical properties of bacterial cellulose composites. Carbohydr. Polym. 2017, 174, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Torres, C.A.; Reis, M.A. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Vu, C.M.; Nguyen, D.D.; Sinh, L.H.; Pham, T.D.; Pham, L.T.; Choi, H.J. Environmentally benign green composites based on epoxy resin/bacterial cellulose reinforced glass fiber: Fabrication and mechanical characteristics. Polym. Test. 2017, 61, 150–161. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, G.; Xu, Y.; Yu, Y.; Wu, J.; Zou, B. High hydrostatic pressure and co-fermentation by Lactobacillus rhamnosus and Gluconacetobacter xylinus improve flavor of yacon-litchi-longan juice. Foods 2019, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, A.; Roy, D.; LaPointe, G. Enhanced exopolysaccharide production by Lactobacillus rhamnosus in co-culture with Saccharomyces cerevisiae. Appl. Sci. 2019, 9, 4026. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Catchmark, J.M. Bacterial cellulose/hyaluronic acid nanocomposites production through co-culturing Gluconacetobacter hansenii and Lactococcus lactis under different initial ph values of fermentation media. Cellulose 2020, 27, 2529–2540. [Google Scholar] [CrossRef]
- Ding, R.; Hu, S.; Xu, M.; Hu, Q.; Jiang, S.; Xu, K.; Tremblay, P.-L.; Zhang, T. The facile and controllable synthesis of a bacterial cellulose/polyhydroxybutyrate composite by co-culturing Gluconacetobacter xylinus and Ralstonia eutropha. Carbohydr. Polym. 2021, 252, 117137. [Google Scholar] [CrossRef]

| Biocatalyst [Reference]; Features of Cells | Substrate Specificity of BTCs and Main Product (PS) | Conditions for BTCs’ Use | Rate of PS Synthesis |
|---|---|---|---|
| Dextran | |||
| Leuconostoc mesenteroides SF3 [33] | Sucrose-100.0 g/L Dextran-22.5 g/L | pH 6.5, 30 °C, 20 h | 1.13 g/L/h |
| L. mesenteroides SF3 [34] | Sucrose-100.0 g/L Dextran-20.8 g/L | pH 6.0, 28 °C, 24 h | 0.87 g/L/h |
| Weissella confusa R003 [35] | Sucrose-100.0 g/L/ Dextran-25.0 g/L | pH 7.5, 30 °C, 24 h, 125 rpm | 1.04 g/L/h |
| L. mesenteroides N7 [36] | Sucrose 100.0 g/L/ Dextran-13.2 g/L | 30 °C, 24 h, 100 rpm | 0.55 g/L/h |
| L. citreum B-2 [37] | Sucrose 75.0 g/L/ Dextran-28.3 g/L | 30 °C, 48 h, 80 rpm | 0.59 g/L/h |
| L. pseudomesenteroides XG5 [38] | Sucrose-125.0 g/L/ Dextran-35.5 g/L | 30 °C, 48 h | 0.74 g/L/h |
| L. pseudomesenteroides G29 [39] | Sucrose-101.4 g/L/ Dextran-38.4 g/L | pH 5.5, 30 °C, 10 h, 200 rpm | 3.84 g/L/h |
| W. cibaria 27 [40] | Sucrose-60.0 g/L Dextran-24.8 | pH 6.2, 22 °C,24 h | 1.03 g/L/h |
| W. confusa Ck15 [41] | Sucrose-20.0 g/L Chickpea flour-280.0 g/L Dextran-14.9 g/L | pH 6.3, 30 °C, 24 h | 0.62 g/L/h |
| L. mesenteroides NRRL B-512F [42] | Sucrose-200.0 g/L Milk permeate powder-150.0 g/L Dextran-42.9 g/L | 30 °C, 24 h | 1.79 g/L/h |
| L. mesenteroides MTCC 7337 [43] | Sugars in sugarcane juice-50.0 g/L Dextran-14.3 g/L | pH 7.0, 30 °C, 72 h, 150 rpm | 0.20 g/L/h |
| L. pseudomesenteroides DSM20193 [44] | Sucrose-40.0 g/L Brewers’ spent grain-100.0 g/L Dextran-11.1 g/L | pH 6.4, 25 °C, 24 h, 200 rpm | 0.46 g/L/h |
| Lactobacillus mali CUPV271 [45] | Sucrose-20 g/L/ Dextran-11.7 g/L | pH 5.5; 28 °C; 48 h | 0.24 g/L/h |
| Weissella cibaria 10 M; mutant strain [46] | Sucrose-171.0 g/L Dextran-14.0 g/L | pH 5.4–6.2, 25 °C, 24 h | 0.58 g/L/h |
| L. mesenteroides KIBGE-IB22M20; mutant strain [47] * | Sucrose-250.0 g/L Dextran-10.5 g/L | pH 7.5, 25 °C, 12 h | 0.88 g/L/h |
| L. mesenteroides subsp. dextranicum B-5481; immobilized in PVA cryogel [48] | Sucrose-200.0 g/L/ Dextran-63.0 g/L | pH 7.0, 28 °C, 15 h, 200 rpm | 4.20 g/L/h; reuse in 5 cycles |
| L. mesenteroides KIBGE HA1 [49]; immobilized in Ca-alginate gel | Sucrose-100.0 g/L Dextran-8.0 g/L | pH 5.0, 30 °C, 24 h 200 rpm | 0.33 g/L/h; reuse in 12 cycles |
| Levan | |||
| Bacillus subtilis MTCC 441 [50] | Sucrose-100.0 g/L Levan-30.4 g/L | pH 7.0, 37 °C, 20 h, 150 rpm | 1.52 g/L/h |
| Z. mobilis PTCC 1718 [51] | Sucrose-300.0 g/L Levan-57.0 g/L | 28 °C, 48 h | 1.19 g/L/h |
| Bacillus sp. MTCC 1434 [52] | Sucrose-250.0 g/L Levan-61.0 g/L | pH 6.0, 30 °C, 30 h, 100 rpm | 2.03 g/L/h |
| Brachybacterium phenoliresistens KX139300 [53] | Sucrose-300.0 g/L Levan-8.6 g/L | pH 7.8, 30 °C, 72 h, 150 rpm | 0.12 g/L/h |
| B. subtilis (NCIM 5021) [50] | Fresh coconut inflorescence sap (sugars g/L: sucrose–172.3 glucose-16.2, fructose-6.2) Levan-62.1 g/L | pH 6.5, 35 °C, 17 h, 150 rpm | 3.65 g/L/h |
| Zymomonas mobilis CCT4494; immobilized in PVA cryogel [54] | Sucrose-300.0 g/L Levan-81.2 g/L | pH 7.0, 30 °C, 12 h | 6.77 g/L/h |
| Z. mobilis CCT4494; immobilized in Ca-alginate gel [55] | Sucrose-350.0 g/L Levan-21.1 g/L | pH 4.0, 30 °C, 24 h, 200 rpm | 0.88 g/L/h; reuse in 12 cycles |
| Z. mobilis CCT4494; immobilized on sugarcane bagasse [56] | Sucrose-350.0 g/L Levan-32.1 g/L | pH 4.0, 30 °C, 24 h | 1.34 g/L/h; reuse in 12 cycles |
| Xanthan | |||
| Xanthomonas campestris AM001 [57] | Maltose-70.0 g/L Xanthan-40.7 g/L | pH 7.0, 32 °C, 80 h, 600 rpm | 0.51 g/L/h |
| X. campestris ATCC 13951 [58] | Winery wastewater (sugars g/L-30.7) Xanthan-23.9 g/L | pH 7.0, 29 °C, 96 h, 475 rpm | 0.25 g/L/h |
| X. campestris pv. campestris 1866 and 1867 [59] | Coconut shells or cocoa husks hydrolysates-20.0 g/L (25.0 g/L of sugars) Xanthan-3.6 g/L (coconut shells) Xanthan-4.5 g/L (cocoa husks) | 28 °C, 96 h, 250 rpm | 0.04–0.05 g/L/h |
| X. campestris WXLB-006; mutant strain [60] | Glycerol-40.0 g/L (+fed batch 1–3 g/L/h) Xanthan-33.9 g/L | pH 7.0, 30 °C, 60 h, 200 rpm | 0.57 g/L/h |
| X. campestris ATCC 13951; immobilized in polyurethane foam [61] | Sucrose-50.0 g/L/ Xanthan-59.9 g/L | 28 °C, 96 h, 180 rpm | 0.62 g/L/h; reuse in 12 cycles |
| X. campestris PTCC 1473; self-immobilized cells on stainless-steel support [62] | Glucose-20.0 g/L/ Xanthan-3.5 g/L | pH-6.9, 30 °C, 47 h, 180 rpm | 0.08 g/L/h |
| X. campestris PTCC1473; immobilized in calcium alginate–polyvinyl alcohol-boric acid gel [63] | Hydrolyzed starch-20.0 g/L Xanthan-9.2 g/L | pH 6.6, 28 °C, 48 h, 180 rpm | 0.19 g/L/h; reuse in 3 cycles |
| X. campestris PTCC 1473; immobilized on plastic (polyethylene) support [64] | Glucose-20.0 g/L Xanthan-8.0 g/L | pH 7.2, 30 °C, 48 h, 180 rpm | 0.17 g/L/h |
| Alginate | |||
| Pseudomonas stutzeri [65] | Sucrose-20.0 g/L Alginate-5.0 g/L | pH 7.0, 30 °C, 600 h, 200 rpm | 0.008 g/L/h |
| Azotobacter vinelandii 12 [66] | Sucrose-35.0 g/L Alginate-2.7 g/L | pH 7.2, 28 °C, 72 h, 210 rpm | 0.04 g/L/h |
| A. vinelandii, NRRL-14641 [67] | Apple peels-10.0 g/ Alginate-180.6 mg/g | pH 7.5, 38 °C, 48 h | 3.76 mg/g/h. |
| A. vinelandii AT9; mutant strain [68] | Sucrose-20.0 g/L Alginate-3.8 g/L | pH 7.2, 29 °C, 72 h, 200 rpm | 0.05 g/L/h |
| A. vinelandii B10436; immobilized in PVA cryogel [22] | Sucrose-30.0 g/L Alginate-2.5 g/L | pH 7.0, 29 °C, 72 h, 200 rpm | 0.035 g/L/h; reuse in 5cycles |
| Pullulan | |||
| Aureobasidium melanogenum TN1-2 [69] | Sucrose-140.0 g/L Pullulan-114.0 g/L | 28 °C, 132 h, 250 rpm | 0.86 g/L/h |
| Rhodosporidium paludigenum PUPY-06 [70] | Sucrose-50.0 g/L Pullulan-21.0 g/L | pH 6.0, 25 °C, 168 h, 150 rpm | 0.125 g/L/h |
| A. melanogenum A4 [71] | Maltose-303.0 g/L/ Pullulan-122.3 g/L | pH 7.0, 30 °C, 120 h, 180 rpm | 1.02 g/L/h |
| A. pullulans Y-4137; immobilized in PVA cryogel [15] | Hydrolysate of Jerusalem artichoke tubers, hydrolysate of potato pulp, hydrolysate of Chlorella vulgaris biomass (glucose-15.0–25.0 g/L) Pullulan-3.5–16.8 g/L | pH 5.5, 26 °C, 50 h, 200 rpm | 0.07–0.33 g/L/h reuse in 15 cycles |
| Bacterial cellulose (BC) | |||
| Lactobacillus hilgardii IITRKH159 [72] | Fructose-50.0 g/L BC-7.2 g/L | 28 °C, 384 h | 0.02 g/L/h |
| Komagataeibacter maltaceti [73] | Dextrin-8.0 g/L BC-6.5 g/L | pH 6.0, 30 °C, 134 h | 0.05 g/L/h |
| K. nataicola [73] | Maltose-10.0 g/L BC-5.4 g/L | pH 6.0, 30 °C, 134 h | 0.04 g/L/h |
| K. rhaeticus [74] | Acerola waste hydrolysate- 50.0 g/L + glucose 20.0 g/L BC-2.3 g/L | pH 3.6, 30 °C, 288 h | 0.008 g/L/h |
| Medusomyces gisevii Sa-12 [75] | Miscanthus biomass hydrolysate (sugars-20.1–21.2 g/L) BC-1.24 g/L | pH 4.0–4.6, 27 °C, 288 h | 0.004 g/L/h |
| Gluconacetobacter xylinus CGMCC 2955; mutant strain [76] | Glucose-25.0 g/L BC-4.3 g/L | pH 6.0, 30 °C, 360 h | 0.012 g/L/h |
| K. xylinum; immobilized in PVA cryogel [32] | Glycerol-20.0 g/L BC-2.8 g/L | pH 7.0, 28 °C, 168 h | 0.017 g/L/h |
| Aspen sawdust hydrolysate (sugars-42.0 g/L) BC-2.9 g/L | 0.017 g/L/h | ||
| Wheat straw hydrolysate (sugars-38.0 g/L) BC-3.6 g/L | 0.021 g/L/h | ||
| Rice straw hydrolysate (sugars-40.0 g/L) BC-3.5 g/L | 0.021 g/L/h | ||
| Jerusalem artichoke tubers hydrolysate (sugars-53.0 g/L) BC-4.5 g/L | 0.027 g/L/h | ||
| Chlorella vulgaris C1 biomass hydrolysate (sugars-45.1 g/L) BC-2.6 g/L | 0.015 g/L/h | ||
| Laminaria saccharina biomass hydrolysate (sugars-36.6 g/L) BC-0.07 g/L | 0.0004 g/L/h | ||
| Acanthophora muscoide biomass hydrolysate (sugars-56.0 g/L BC-0.4 g/L | 0.002 g/L/h | ||
| Ulva lactuca biomass hydrolysate (sugars-24.1 g/L) BC-0.08 g/L | 0.0005 g/L/h reuse in 10 cycles | ||
| Fructo-oligosaccharides | |||
| A. pullulans FRR 5284 [77] | Sucrose-500.0 g/L Fructo-oligosaccharides-306.3 g/L | pH 5.5, 55 °C, 3 h | 102.1 g/L/h |
| A. pullulans CCY 27-1-94; immobilized on reticulated polyurethane foam [78] | Sucrose-200.0 g/L Fructo-oligosaccharides-108.2 g/L | pH 5.5, 28 °C, 25 h, 150 rpm | 4.33 g/L/h |
| A. pullulans CCY 27-1-94; immobilized on walnut shell [78] | Sucrose-200.0 g/L Fructo-oligosaccharides-126.5 g/L | pH 5.5; 28 °C; 36 h, 150 rpm | 3.51 g/L/h |
| Curdlan | |||
| Bacillus cereus PR3 [79] | Starch-100.0 g/L Curdlan-20.9 g/L | 30 °C, 96 h, 200 rpm | 0.22 g/L/h |
| Paenibacillus sp. NBR-10 [80] | Sucrose-50.0 g/L Curdlan-4.8 g/L | pH 7.0, 35 °C, 84 h, 200 rpm | 0.06 g/L/h |
| Agrobacterium sp. ATCC 13140 [81] | Juice of discarded asparagus– 100 g/L (Sucrose-50.0 g/L) Curdlan-40.2 g/L | pH 5.5, 30 °C, 168 h, 200 rpm | 0.24 g/L/h |
| Agrobacterium sp. DH-2 [82] | Cassava starch hydrolysate (sugars-90 g/L) Curdlan-28.4 g/L | pH 5.5, 30 °C, 96 h, 250 rpm | 0.30 g/L/h |
| Agrobacterium sp. CGMCC 11546; mutant strain [83] | Sucrose-60.0 g/L Curdlan-48.0 g/L | pH 5.0, 30 °C, 96 h, 280 rpm | 0.50 g/L/h |
| Agrobacterium sp. IFO 13140; immobilized on loofa sponge [84]; | Glucose-50.0 g/L Curdlan-17.8 g/L | pH 6.5, 30 °C, 240 h, 150 rpm | 0.07 g/L/h; reuse in 5 cycles |
| Succinoglycan | |||
| Rhizobium radiobacter ATCC4720 [85] | Rice husk hydrolysate-100.0 g/L Succinoglycan-69.0 g/L | pH 7.0, 30 °C, 72 h, 100 rpm | 0.96 g/L/h |
| R. radiobacter ATCC4720 [86] | Deproteinized whey–50.0 g/L Succinoglycan-13.7 g/L | pH 7.0, 30 °C, 192 h,180 rpm | 0.07 g/L/h |
| R. radiobacter 18052 N-11; mutant strain [87] | Sucrose-70.0 g/L Succinoglycan-32.5 g/L | pH 7.2, 30 °C, 72 h, 250 rpm | 0.45 g/L/h |
| Agrobacterium radiobacter NBRC 12665; immobilized on loofa sponge [88] | Sugarcane molasses-75.0 g/L Succinoglycan-14.1 g/L | pH 7.0, 30 °C, 192 h, 180 rpm | 0.07 g/L/h; reuse in 5 cycles |
| Biocatalysts [Reference] | Substrate Product Concentrations | Conditions of BTCs Use | * Rate of Biocatalysis Provided by BTCs |
|---|---|---|---|
| Consortia | |||
| Consortia Kombucha (Komagataeibacter saccharivorans LN886705, Brettanomyces bruxellensis MH393498, Brettanomyces anomalus KY103303) [91] | Glucose-60 g/L BC-18.7 g/L | pH 6, 30 °C, 240 h | 0.078 g/L/h |
| Symbiotic community of bacteria and yeast cells (SCOBY) [92] | Sucrose-10 g/L BC-22 g/L | 26 °C, 336 h | 0.065 g/L/h |
| Consortia Kombucha [93] | Sucrose-110 g/L BC-10.6 g/L | 22 °C, 504 h | 0.021 g/L/h |
| Co-culturing | |||
| Agrobacterium sp. ATCC 31749 and Trichoderma harzianum GIM 3.442 [94] | Glucose-50.0 g/L Curdlan-47.9 g/L | pH 7.5, 37 °C, 96 h | 0.33 g/L/h |
| “Step-by-step” culturing | |||
| A. pullulans FRR 5284 and invertase-free Saccharomyces cerevisiae 1403 cells [77] | Molasses-300 g/L Fructo-oligosaccharides-169.5 g/L | pH 5.5, 55 °C, 1 h | 169.5 g/L/h |
| Biocatalysts [Reference] | Substrate for BTCs; Additives for NCs Formation; NCs as Products | Conditions for BTCs Use; * Rate of PS Synthesis | Possible Applications |
|---|---|---|---|
| Introduction of additives into the medium during the synthesis of PS by monoculture | |||
| Klebsiella oxytoca SK01 [121] | Ferric citrate-50 Mm; Composite of EPS with Ferrihydrite (5Fe2O3 × 9H2O) as nanoparticles (1.8 nm); EPS attached to the cell surface and containing galactose, glucuronic acid, and rhamnose that display metal-binding properties. | pH 7.4 25 °C 336 h | Biomedical and farming industries: tissue engineering, diagnostic magnetic resonance imaging, DNA detection, intracellular labeling, magnetic transfections, anti-proliferative hyperthermia therapy, targeted drug delivery systems |
| Natural consortium from apple biomass fermentation [122] | Sucrose-110 g/L Graphene oxide (GO)-50 mg/L; Composite of BC with GO with high water content (~400%), cellulose nanofiber ~100 nm. Composite BC/GO with high sorption capacity (60 mg paracetamol/g BC) | pH 3.5 25 °C 240 h | Composites for the creation of dressings with prolonged release of medicinal substances |
| Komagataeibacter xylinus ATCC 11142 [123] | Glucose from sugar cane straw fermentolysate-20 g/L Reduced graphene oxide (RGO)- 2 wt% Nanocomposite of BC with RGO The partial oxidation of GO in presence of peroxides resulted in incorporation of hydroxyl and epoxy groups in RGO. | pH 6.5 30 °C 360 h 0.0125 g/L/h | Electrodes/supercapacitors, adsorbents, scaffolds for tissue regeneration |
| G. xylinus KCCM 40,216 [124] | Mannitol-2.5% w/w Multi-walled CNT-dispersed carbon tubes-0.001% (w/v) Composite of BC with Carbon nanotubes (CNTs) with pore size-85.4 ± 2.8 µm. | pH 6.0 28 °C 336 h | Functional nanomaterials for bone regeneration with high efficiency in vivo, promoting osteogenesis of mesenchymal stem cells, bone calcification |
| G. xylinus CGMCC 2955 [125] | Glucose-20 g/L Oriented glass fiber-20 wt% Composite of BC-glass fiber (with a diameter between 7–15 µm) | pH 6.0 30 °C 72 h electric field of 10 mA | Materials for cell growth and subsequent tissue organization |
| Acetobacter xylinum ATCC 53582 [126] | Glucose-5.5 g/L, PVA-6% Composite of BC with PVA with further modification by genipin-crosslinked silk sericin (SS) and azithromycin (AZM) | 30 °C 192 h | Materials for biomedical and farming industries with inhibition and disruption of biofilms due to AZM action |
| Komagataeibacter hansenii ATCC 23769 (previously known as Gluconacetobacter hansenii) [127] | Glucose-50 g/L Pullulan-15 g/L Composite of BC with pullulan | pH 5.0 30 °C 200 rpm 168 h 0.005 dry g/L/h | Materials for food packaging, transparent organic electronic carriers, biomedical material |
| Consolidated bioprocess | |||
| Co-culture of Gluconacetobacter hansenii ATCC 23769 with Escherichia coli ATCC 700728 [128] | Glucose-20 g/L Composite of BC with EPS | pH 5.5 30 °C 120 h 0.013 dry g/L/h | Materials for medicine: artificial skin, vessels, grafts, cardiovascular implants |
| Co-culture of Komagataeibacter hansenii ATCC 23769 (previously known as Gluconacetobacter hansenii) with Aureobasidium pullulans ATCC 201,253 [118,127] | Glucose-50 g/L Composite of BC with pullulan | pH 5.0 30 °C 200 rpm 168 h 0.002 dry g/L/h | Materials for food packaging, transparent organic electronic carriers, biomedical material |
| Komagataeibacter xylinus C3 and Aureobasidium pullulans C7 [129] | Molasses–20 g/L Composite of BC with pullulan-16.8 g/L | pH 6 30 °C 168 h 0.1 g/L/h | Matrix for obtaining a “synthetic synbiotic” consortia for testing in vitro and in vivo, an effective cell delivery system to the host body; the basis for the creation of synbiotic dairy products with a preventive effect; matrix for immobilized probiotic cultures of microorganisms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in Synthesis of Microbial Polysaccharides: Properties and Development Trends. Catalysts 2022, 12, 1377. https://doi.org/10.3390/catal12111377
Efremenko E, Senko O, Maslova O, Stepanov N, Aslanli A, Lyagin I. Biocatalysts in Synthesis of Microbial Polysaccharides: Properties and Development Trends. Catalysts. 2022; 12(11):1377. https://doi.org/10.3390/catal12111377
Chicago/Turabian StyleEfremenko, Elena, Olga Senko, Olga Maslova, Nikolay Stepanov, Aysel Aslanli, and Ilya Lyagin. 2022. "Biocatalysts in Synthesis of Microbial Polysaccharides: Properties and Development Trends" Catalysts 12, no. 11: 1377. https://doi.org/10.3390/catal12111377