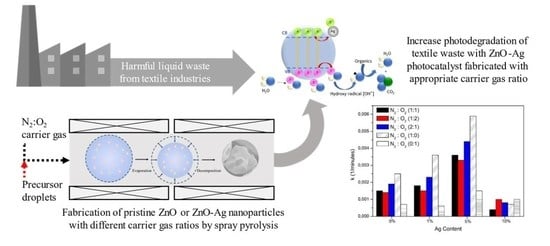
Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Analysis of the Produced Nanoparticles
2.2. Analysis of the Crystal Structure of the Produced Nanoparticles
2.3. Surface Area of the Produced Nanoparticles
2.4. Photocatalytic Activity of Pristine ZnO and ZnO-Ag at Different Carrier Gas Ratios
3. Materials and Methods
3.1. Preparation Methods
3.2. Materials Characterization
3.3. Photocatalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, N.; Gupta, V.; Thirumal, E.; Thangadurai, P.; Narayanan, V.; Stephen, A. ZnO/Ag nanocomposite: An efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C 2013, 33, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Amara, A.B.H.; Ruiz-Hitzky, E. ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 156, 104–109. [Google Scholar] [CrossRef]
- Kusdianto, K.; Hudandini, M.; Kusuma, T.C.; Widiyastuti, W.; Madhania, S.; Machmudah, S.; Nurtono, T.; Puspitasari, D.; Winardi, S. Photocatalytic degradation of organic waste derived from textile dye by ZnO-Ag nanocomposite synthesized by spray pyrolysis. AIP Conf. Proc. 2020, 2219, 30001. [Google Scholar]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; El-Marsafy, S.M.; El-Maddah, A.A. Enhancement of the photocatalytic activity of ZnO nanoparticles by silver doping for the degradation of AY99 contaminants. J. Mol. Struct. 2019, 1191, 76–84. [Google Scholar] [CrossRef]
- Kusdianto, K.; Sari, T.D.; Laksono, M.A.; Madhania, S.; Winardi, S. Fabrication and application of ZnO-Ag nanocomposite materials prepared by gas-phase methods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 12023. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Lee, Y.; Fujimoto, T.; Yamanaka, S.; Kuga, Y. Evaluation of photocatalysis of Au supported ZnO prepared by the spray pyrolysis method. Adv. Powder Technol. 2021, 32, 1619–1626. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Sierra-Fernandez, A.; Milošević, O.; Rabanal, M.E. Solvothermal synthesis of Ag/ZnO and Pt/ZnO nanocomposites and comparison of their photocatalytic behaviors on dyes degradation. Adv. Powder Technol. 2016, 27, 983–993. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Xu, M. Ag-decorated ZnO-based nanocomposites for visible light-driven photocatalytic degradation: Basic understanding and outlook. J. Phys. D Appl. Phys. 2022, 55, 483001. [Google Scholar] [CrossRef]
- Dermenci, K.B.; Genc, B.; Ebin, B.; Olmez-Hanci, T.; Gürmen, S. Photocatalytic studies of Ag/ZnO nanocomposite particles produced via ultrasonic spray pyrolysis method. J. Alloys Compd. 2014, 586, 267–273. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, F. Nanoparticle processing for optical applications—A review. Adv. Powder Technol. 2009, 20, 283–292. [Google Scholar] [CrossRef]
- Jongthammanurak, S.; Witana, M.; Cheawkul, T.; Thanachayanont, C. The effects of carrier gas and substrate temperature on ZnO films prepared by ultrasonic spray pyrolysis. Mater. Sci. Semicond. Process. 2013, 16, 625–632. [Google Scholar] [CrossRef]
- Wittawat, R.; Rittipun, R.; Jarasfah, M.; Nattaporn, B. Synthesis of ZnO/TiO2 spherical particles for blue light screening by ultrasonic spray pyrolysis. Mater. Today Commun. 2020, 24, 101126. [Google Scholar] [CrossRef]
- Vorovsky, V.Y.; Kovalenko, A.V.; Kushneryov, A.I.; Khmelenko, O.V. Preparation of zinc oxide nanopowders doped with manganese, which have ferromagnetic properties at room temperature. Funct. Mater. 2018, 25, 61–66. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Cheng, Y.; Li, Z.; Cheng, Y.; Feng, Q.; Chen, D.; Zhang, J.; Hao, Y. Influence of Carrier Gases on the Quality of Epitaxial Corundum-Structured α-Ga2O3 Films Grown by Mist Chemical Vapor Deposition Method. Matererials 2019, 12, 3670. [Google Scholar] [CrossRef] [Green Version]
- Rahemi Ardekani, S.; Sabour Rouhaghdam, A.; Nazari, M. N-doped ZnO-CuO nanocomposite prepared by one-step ultrasonic spray pyrolysis and its photocatalytic activity. Chem. Phys. Lett. 2018, 705, 19–22. [Google Scholar] [CrossRef]
- El Rouby, W.M.A. Crumpled graphene: Preparation and applications. RSC Adv. 2015, 5, 66767–66796. [Google Scholar] [CrossRef]
- Wang, R.-C.; Tsai, C.-C. Efficient synthesis of ZnO nanoparticles, nanowalls, and nanowires by thermal decomposition of zinc acetate at a low temperature. Appl. Phys. A 2009, 94, 241–245. [Google Scholar] [CrossRef]
- Kusdianto, K.; Hudandini, M.; Jiang, D.; Kubo, M.; Shimada, M. Effect of Heating Rate on the Photocatalytic Activity of Ag–TiO2 Nanocomposites by One-Step Process via Aerosol Routes. Catalysts 2021, 12, 17. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.-H.; Zhang, Y.-L.; Yang, Y.; Song, W.-J.; Li, X.-M. Effects of rapid thermal annealing in different ambients on structural, electrical, and optical properties of ZnO thin films by sol-gel method. J. Electroceramics 2011, 26, 84–89. [Google Scholar] [CrossRef]
- Yan, Y.; Al-Jassim, M.M.; Wei, S.H. Doping of ZnO by group-IB elements. Appl. Phys. Lett. 2006, 89, 181912. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Kuk, H.; Lee, D.H.; Hahn, Y.-B.; Khang, G. Rapid methyl orange degradation using porous ZnO spheres photocatalyst. J. Photochem. Photobiol. B Biol. 2016, 161, 312–317. [Google Scholar] [CrossRef]
- Ma, H.; Yue, L.; Yu, C.; Dong, X.; Zhang, X.; Xue, M.; Zhang, X.; Fu, Y. Synthesis, characterization and photocatalytic activity of Cu-doped Zn/ZnO photocatalyst with carbon modification. J. Mater. Chem. 2012, 22, 23780–23788. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A Highly Efficient Ag-ZnO Photocatalyst: Synthesis, Properties, and Mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef] [Green Version]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Effect of annealing temperature on the photocatalytic activity of Ag–TiO2 nanocomposite films by one-step gas-phase deposition. Mater. Res. Bull. 2018, 97, 497–505. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudandini, M.; Puri, N.R.; Winardi, S.; Widiyastuti, W.; Shimada, M.; Kusdianto, K. Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents. Catalysts 2022, 12, 1374. https://doi.org/10.3390/catal12111374
Hudandini M, Puri NR, Winardi S, Widiyastuti W, Shimada M, Kusdianto K. Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents. Catalysts. 2022; 12(11):1374. https://doi.org/10.3390/catal12111374
Chicago/Turabian StyleHudandini, Meditha, Nurdiana Ratna Puri, Sugeng Winardi, Widiyastuti Widiyastuti, Manabu Shimada, and Kusdianto Kusdianto. 2022. "Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents" Catalysts 12, no. 11: 1374. https://doi.org/10.3390/catal12111374