Interfacial Electronic Rearrangement and Synergistic Catalysis for Alkaline Water Splitting in Carbon-Encapsulated Ni (111)/Ni3C (113) Heterostructures
Abstract
:1. Introduction
2. Results
2.1. Structural and Composition Characterization of Ni-Ni3C/NC
2.2. Electrochemical HER of Ni-Ni3C/NC
2.3. Electrochemical OER and OWS of Ni-Ni3C/NC


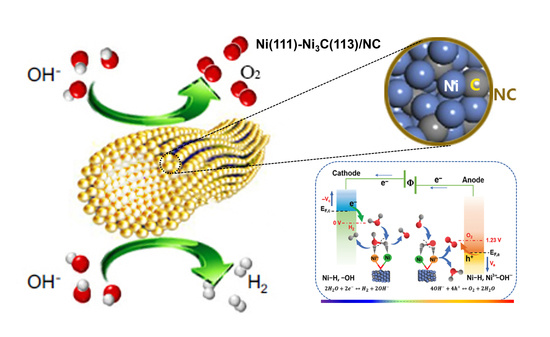
2.4. Interfacial Electronic Rearrangement and the Heterostructure’s Synergistic Catalysis for HER and OER

3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ni-NTA Nanorods
3.3. Synthesis of Ni-Ni3C/NC Porous Nanorods
3.4. Characterization
3.5. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Qiu, C.; Wang, S.; Gao, H.; Yu, K.; Zhang, Z.; Ling, X.; Ou, W.; Su, C. Electrocatalytic water-splitting for the controllable and sustainable synthesis of deuterated chemicals. Sci. Bull. 2021, 66, 562–569. [Google Scholar] [CrossRef]
- Gandía, L.M.; Oroz, R.; Ursúa, A.; Sanchis, P.; Diéguez, P.M. Renewable Hydrogen Production: Performance of an Alkaline Water Electrolyzer Working under Emulated Wind Conditions. Energy Fuels 2007, 21, 1699–1706. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Huang, F.; Zhang, H.; Li, S. Hierarchical Cu(OH)2@Ni2(OH)2CO3 core/shell nanowire arrays in situ grown on three-dimensional copper foam for high-performance solid-state supercapacitors. J. Mater. Chem. A 2017, 5, 9960–9969. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, F.; Yao, Y.; Jiao, L. Crystalline Ni(OH)2/Amorphous NiMoOx Mixed-Catalyst with Pt-Like Performance for Hydrogen Production. Adv. Energy Mater. 2019, 9, 1902703. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, Z.; Huang, J.; Gao, F. Fe-doped NiO mesoporous nanosheets array for highly efficient overall water splitting. J. Catal. 2018, 358, 243–252. [Google Scholar] [CrossRef]
- Sun, Q.; Dong, Y.; Wang, Z.; Yin, S.; Zhao, C. Synergistic Nanotubular Copper-Doped Nickel Catalysts for Hydrogen Evolution Reactions. Small 2018, 14, e1704137. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Yao, Y.; Huang, X.; Willinger, M.; Shao, L. Ni/NiO nanoparticles on a phosphorous oxide/graphene hybrid for efficient electrocatalytic water splitting. J. Mater. Chem. A 2017, 5, 14758–14762. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, T.; Liu, P.; Liu, S.; Dong, R.; Zhuang, X.; Chen, M.; Feng, X. Engineering water dissociation sites in MoS2 nanosheets for accelerated electrocatalytic hydrogen production. Energy Environ. Sci. 2016, 9, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Liu, J.; Wang, J.; Ruan, Y.; Ji, X.; Xu, K.; Chen, C.; Wan, H.; Miao, L.; Jiang, J. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution. Nano Energy 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Lin, H.; Shi, Z.; He, S.; Yu, X.; Wang, S.; Gao, Q.; Tang, Y. Heteronanowires of MoC-Mo2C as efficient electrocatalysts for hydrogen evolution reaction. Chem. Sci. 2016, 7, 3399–3405. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.; Xiao, J.; Bian, X.; Zhang, Y.; Scanlon, M.D.; Hu, X.; Tang, Y.; Liu, B.; Girault, H.H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jiang, J.; Ding, T.; Chen, G.; Xu, W.; Yang, Q. Monodisperse Ternary NiCoP Nanostructures as a Bifunctional Electrocatalyst for Both Hydrogen and Oxygen Evolution Reactions with Excellent Performance. Adv. Mater. Interfaces 2016, 3, 1500454. [Google Scholar] [CrossRef]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Ren, Z.; Kong, L.; Wu, J.; Du, S.; Zhu, J.; Xue, Y.; Meng, H.; Fu, H. Dual-valence nickel nanosheets covered with thin carbon as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2016, 4, 7297–7304. [Google Scholar] [CrossRef]
- Conway, B.E.; Jerkiewicz, G. Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics. Electrochim. Acta 2000, 45, 4075–4083. [Google Scholar] [CrossRef]
- Xiao, P.; Sk, M.A.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.Y.; Lim, K.H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, S.; Li, Y.; Jacob, R.J.; Kuang, Y.; Liu, B.; Wang, Y.; Pastel, G.; Salamanca-Riba, L.G.; Zachariah, M.R.; et al. FeS2 Nanoparticles Embedded in Reduced Graphene Oxide toward Robust, High-Performance Electrocatalysts. Adv. Energy Mater. 2017, 7, 1700482. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.; Li, Y.; Dong, H.; Sun, D.; Liu, X.; Xu, L.; Tian, Z.; Tang, Y. Regulating the Electronic Structure of CoP Nanosheets by O Incorporation for High-Efficiency Electrochemical Overall Water Splitting. Adv. Funct. Mater. 2019, 30, 1905252. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni-Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Jain, A.; Castelli, I.E.; Hautier, G.; Bailey, D.H.; Jacobsen, K.W. Performance of genetic algorithms in search for water splitting perovskites. J. Mater. Sci. 2013, 48, 6519–6534. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Humayun, M.; Zhang, H.; Bo, Y.; Ao, X.; Xu, X.; Chen, K.; Ostrikov, K.; Huo, K.; et al. Achieving highly efficient pH-universal hydrogen evolution by superhydrophilic amorphous/crystalline Rh(OH)3/NiTe coaxial nanorod array electrode. Appl. Catal. B Environ. 2022, 305, 121088. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.T.; Myers, D.; et al. Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew. Chem. Int. Ed. 2014, 53, 14016–14021. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Zhang, B.; Hu, Y.; Wang, D.Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Zhou, W.; Kenney, M.J.; Kapusta, R.; Cowley, S.; Wu, Y.; Lu, B.; Lin, M.C.; Wang, D.Y.; Yang, J.; et al. Blending Cr2O3 into a NiO-Ni electrocatalyst for sustained water splitting. Angew. Chem. Int. Ed. 2015, 54, 11989–11993. [Google Scholar] [CrossRef]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3 C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, e2001642. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, X.; Cao, Y.; Chen, H.S.; Yang, P. Ni/Ni3C core-shell nanoparticles encapsulated in N-doped bamboo-like carbon nanotubes towards efficient overall water splitting. Inorg. Chem. Front. 2019, 6, 1073–1080. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, Q.; Guo, Z.; Li, H.; Zhao, R.; Chen, Q.; Liu, Z.; Wang, X. Atomic layer deposition of nickel carbide for supercapacitors and electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 4297–4304. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Zou, G.; Yi, Q.; Guo, J.; Gao, L. High-Performance Hydrogen Evolution Electrocatalyst Derived from Ni3C Nanoparticles Embedded in a Porous Carbon Network. ACS Appl. Mater. Interfaces 2017, 9, 60–64. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, Z.; Hu, Z.; Bian, J.; Ye, W.; Wu, T.; Chen, Y.; Gao, P. Abundant hot-spot construction between Ni/C nanotubes with enhanced localized surface plasmon resonance for Radar wave absorption. Appl. Surf. Sci. 2020, 504, 144592. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Analyzing the role of copper in the soot oxidation performance of BaMnO3-perovskite-based catalyst obtained by modified sol-gel synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, S.; Zuo, J.; Zhang, B. Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride. Catalysts 2022, 12, 672. [Google Scholar] [CrossRef]
- Abdullah, F.; Mohd Yusoff, A.R.; Wan Abu Bakar, W.A.; Ismail, R.; Syafiuddin, A. Preparation, characterization, and lead removal appraisal of zinc aluminate prepared at different calcination temperatures. J. Chin. Chem. Soc. 2018, 65, 1199–1209. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized bimetallic phosphides of Ni-Mo, Co-Mo, and Co-Ni in a single ultrathin-3D-nanosheets for efficient HER and OER in alkaline media. Compos. Part B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Han, G.; Liu, X.; Zhang, X.; Sun, Y.; Zhang, M.; Cao, Z.; Sun, Y. Enhanced Electrocatalytic Hydrogen Oxidation on Ni/NiO/C Derived from a Nickel-Based Metal-Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 10644–10649. [Google Scholar] [CrossRef]
- Qin, Q.; Hao, J.; Zheng, W. Ni/Ni3C Core/Shell Hierarchical Nanospheres with Enhanced Electrocatalytic Activity for Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 17827–17834. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Liu, D.; Feng, X.; Shao, M.; Zhang, Y. Ru Nanoparticles Supported on Co-Embedded N-Doped Carbon Nanotubes as Efficient Electrocatalysts for Hydrogen Evolution in Basic Media. Chem. Res. Chin. Univ. 2020, 36, 425–430. [Google Scholar] [CrossRef]
- Cui, X.; Chen, X.; Chen, S.; Jia, F.; Yang, S.; Lin, Z.; Shi, Z.; Deng, H. Dopamine adsorption precursor enables N-doped carbon sheathing of MoS2 nanoflowers for all-around enhancement of supercapacitor performance. J. Alloys Comp. 2017, 693, 955–963. [Google Scholar] [CrossRef]
- Cao, D.; Kang, W.; Huang, Z.; Li, H.; Yang, M.; Li, J.; Gao, Y.; Wang, Y.; Ma, P.; Sun, D. N-doped carbon matrix supported Fe3Ni6S8 hierarchical architecture with excellent sodium storage capability and electrocatalytic properties. Electrochim. Acta 2019, 325, 134925. [Google Scholar] [CrossRef]
- Shang, Y.; Ding, Y.; Zhang, P.; Wang, M.; Jia, Y.; Xu, Y.; Li, Y.; Fan, K.; Sun, L. Pyrrolic N or pyridinic N: The active center of N-doped carbon for CO2 reduction. Chin. J. Catal. 2022, 43, 2405–2413. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, K.; Cheong, W.C.; Zheng, L.; Zhang, C.; Liu, S.; Cao, X.; Wu, K.; Pan, Y.; Zhuang, Z.; et al. Synergistically Interactive Pyridinic-N-MoP Sites: Identified Active Centers for Enhanced Hydrogen Evolution in Alkaline Solution. Angew. Chem. Int. Ed. 2020, 59, 8982–8990. [Google Scholar] [CrossRef]
- Hall, D.S.; Bock, C.; MacDougall, B.; David, L.; Poirier, S. A Spectroscopic and Electrochemical Investigation of the Structure of Ni(OH)2 Materials. ECS Meet. Abst. 2012, MA2012–02, 2208. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Zhang, Y.; Rao, G.; Wu, C.; Hu, Y.; Wang, X.; Lu, R.; Li, Y.; Xiong, J. Identification of Key Reversible Intermediates in Self-Reconstructed Nickel-Based Hybrid Electrocatalysts for Oxygen Evolution. Angew. Chem. Int. Ed. 2019, 58, 17458–17464. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Zheng, L.R.; Tong, Y.; Zhang, M.; Xu, G.; Li, C.; Ma, J.; Shi, G. Hydrogen Evolution Reaction in Alkaline Media: Alpha- or Beta-Nickel Hydroxide on the Surface of Platinum? ACS Energy Lett. 2017, 3, 237–244. [Google Scholar] [CrossRef]
- Patel, K.N.; Deshpande, M.P.; Gujarati, V.P.; Pandya, S.; Sathe, V.; Chaki, S.H. Structural and optical analysis of Fe doped NiO nanoparticles synthesized by chemical precipitation route. Mater. Res. Bull. 2018, 106, 187–196. [Google Scholar] [CrossRef]
- Karishma; Tripathi, N.; Yanagida, M.; Nagataki, A.; Tripathi, A.; Sharma, V. Synthesis and characterization of Na-doped NiO nanocrystals. Mater. Today Proc. 2020, 28, 269–271. [Google Scholar]
- Qiu, Z.; Ma, Y.; Edvinsson, T. In operando Raman investigation of Fe doping influence on catalytic NiO intermediates for enhanced overall water splitting. Nano Energy 2019, 66, 104118. [Google Scholar] [CrossRef]
- Fichtner, C.; Laurich, C.; Bothe, E.; Lubitz, W. Spectroelectrochemical Characterization of the [NiFe] Hydrogenase of Desulfovibrio vulgaris Miyazaki F. Biochemistry 2006, 45, 9706–9716. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, K.; Zhong, H.X.; Xu, D.; Wang, Z.L.; Jiang, Z.; Wu, Z.J.; Zhang, X.B. Synergistic Effect between Metal-Nitrogen-Carbon Sheets and NiO Nanoparticles for Enhanced Electrochemical Water-Oxidation Performance. Angew. Chem. Int. Ed. 2015, 54, 10530–10534. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.Z. A computational study on Pt and Ru dimers supported on graphene for the hydrogen evolution reaction: New insight into the alkaline mechanism. J. Mater. Chem. A 2019, 7, 3648–3654. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, T.; Yang, S. Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 2090–2098. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. In Situ Raman Study of Nickel Oxide and Gold-Supported Nickel Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Phys. Chem. C 2012, 116, 8394–8400. [Google Scholar] [CrossRef] [Green Version]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef] [Green Version]
- Morrow, B.A.; Hardin, A.H. Raman spectra of some hydrogen sequestering agents chemisorbed on silica. J. Phys. Chem. 1979, 83, 3135–3141. [Google Scholar] [CrossRef]
- Qiu, Z.; Tai, C.W.; Niklasson, G.A.; Edvinsson, T. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting. Energy Environ. Sci. 2019, 12, 572–581. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S. Density functional theory with London dispersion corrections. WIREs Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Steinmann, S.N.; Corminboeuf, C. A System-Dependent Density-Based Dispersion Correction. J. Chem. Theory Comput. 2010, 6, 1990–2001. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Tsuboi, M.; Onishi, T.; Nakagawa, I.; Shimanouchi, T.; Mizushima, S.I. Assignments of the vibrational frequencies of glycine. Spectrochim. Acta 1958, 12, 253–261. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano lett. 2009, 9, 1002–1006. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Peng, Z.; Jia, D.; Wang, Y.; Wu, W.; Deng, P.; Xu, M.; Xu, X.; Jia, G.; Ye, W.; et al. Interfacial Electronic Rearrangement and Synergistic Catalysis for Alkaline Water Splitting in Carbon-Encapsulated Ni (111)/Ni3C (113) Heterostructures. Catalysts 2022, 12, 1367. https://doi.org/10.3390/catal12111367
Li X, Peng Z, Jia D, Wang Y, Wu W, Deng P, Xu M, Xu X, Jia G, Ye W, et al. Interfacial Electronic Rearrangement and Synergistic Catalysis for Alkaline Water Splitting in Carbon-Encapsulated Ni (111)/Ni3C (113) Heterostructures. Catalysts. 2022; 12(11):1367. https://doi.org/10.3390/catal12111367
Chicago/Turabian StyleLi, Xiaoyu, Zhenbo Peng, Dongmei Jia, Yikang Wang, Wenbo Wu, Ping Deng, Mengqiu Xu, Xudong Xu, Gan Jia, Wei Ye, and et al. 2022. "Interfacial Electronic Rearrangement and Synergistic Catalysis for Alkaline Water Splitting in Carbon-Encapsulated Ni (111)/Ni3C (113) Heterostructures" Catalysts 12, no. 11: 1367. https://doi.org/10.3390/catal12111367