Ni and Ce Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical–Chemical Characterization
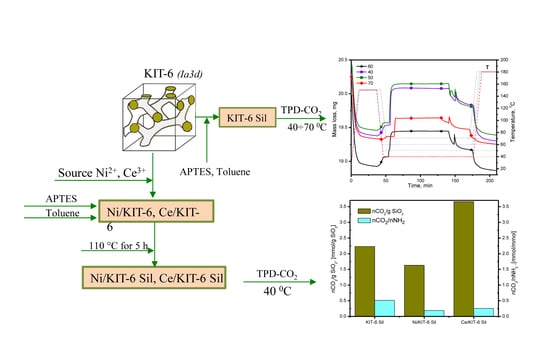
2.2. The Adsorption-Desorption Process CO2
3. Experimental
3.1. Preparation of sample
3.2. Characterization of the Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Yu, X. Carbon dioxide adsorption properties and adsorption/desorption kinetics of amine-functionalized KIT-6. Appl. Energy. 2018, 211, 1080–1088. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Adsorption of CO2-Containing Gas Mixtures over Amine-Bearing Pore-Expanded MCM-41 Silica. Ind. Eng. Chem. Res. 2010, 49, 359–365. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, L.; Chen, S. Rapid synthesis of tunable-structured short-pore SBA-15 and its application on CO2 capture. J. Porous Mater. 2016, 23, 529–537. [Google Scholar] [CrossRef]
- Alreshaidan, S.B.; Ibrahim, A.A.; Fakeeha, A.H.; Almutlaq, A.M.; Ali, F.A.A.; Al-Fatesh, A.S. Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane. Catalysts 2022, 12, 1066. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.; Pinto, F.; Lima, E.; Souza, L.; Caldeira, V.; Santos, A. Influence of Synthesis Parameters in Obtaining KIT-6. Mesoporous Material. Appl. Sci. 2018, 8, 725. [Google Scholar] [CrossRef] [Green Version]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Popa, A.; Sasca, V.; Verdes, O.; Suba, M.; Barvinschi, P. Effect of the amine type on thermal stability of modified mesoporous silica used for CO2 adsorption. J. Therm. Anal. Calorim. 2018, 134, 269–279. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Yang, Y.; Sayari, A. Effect of the Pore Length on CO2 Adsorption over Amine-Modified Mesoporous Silicas. Energy Fuels 2011, 25, 4206–4210. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Zheng, S.; Tao, M.; He, Y.; Shi, Y. Kinetics Studies of CO2 Adsorption/Desorption on Amine-Functionalized Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2014, 53, 11677–11683. [Google Scholar] [CrossRef]
- Oveisi, H.; Anand, C.; Mano, A.; Al-Deyab, S.S.; Kalita, P.; Beitollahi, A.; Vinu, A. Inclusion of size controlled gallium oxide nanoparticles into highly ordered 3D mesoporous silica with tunable pore diameters and their unusual catalytic performance. J. Mater. Chem. A. 2010, 20, 10120–10129. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Pandurangan, A. Post synthesis alumination of KIT-6 materials with Ia3d symmetry and their catalytic efficiency towards multicomponent synthesis of 1H-pyrazolo[1,2-]phthalazine-5,10-dione carbonitriles and carboxylates. J. Mol Catal A Chem. 2012, 361–362, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Kan, Q.; Li, Z.; Yu, G.; Liu, H.; Yang, X.; Guan, J. Different transition metal (Fe2+, Co2+, Ni2+, Cu2+ or VO2+) Schiff complexes immobilized onto three-dimensional mesoporous silica KIT-6 for the epoxidation of styrene. RSC Adv. 2014, 4, 2310–2317. [Google Scholar] [CrossRef]
- Visuvamithiran, P.; Palanichamy, M.; Shanthi, K.; Murugesan, V. Selective epoxidation of olefins over Co(II)-Schiff immobilised on KIT-6. Appl. Catal. A Gen. 2013, 462–463, 31–38. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Akhoundzadeh, H.; Rezayan, A.; Sadeghian, M. Excellent catalytic performance of 3D-mesoporous KIT-6 supported Cu and Ce nanoparticles in methanol steam reforming. Int. J. Hydrog. Energy 2018, 43, 10926–11093. [Google Scholar] [CrossRef]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrog. Energy 2018, 43, 10376–10385. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Wang, X.; Zhao, J.; Zhou, J.; Si, W.; Zhuo, S. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal. Fuel 2018, 233, 377–387. [Google Scholar] [CrossRef]
- Prabhu, A.; Kumaresan, L.; Palanichamy, M.; Murugesan, V. Synthesis and characterization of aluminium incorporated mesoporous KIT-6: Efficient catalyst for acylation of phenol. Appl. Catal. A Gen. 2009, 360, 59–65. [Google Scholar] [CrossRef]
- Sun, H.; Parlett, C.M.A.; Isaacs, M.A.; Liu, X.; Adwek, G.; Wang, J.; Wu, C. Development of Ca/KIT-6 adsorbents for high temperature CO2 capture. Fuel 2019, 235, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Puertolas, B.; Solsona, B.; Agouram, S.; Murillo, R.; Mastral, A.M.; Aranda, A.; Garcia, T. The catalytic performance of mesoporous cerium oxides prepared through a nanocasting route for the total oxidation of naphthalene. Appl. Catal. B Environ. 2010, 93, 395–405. [Google Scholar] [CrossRef]
- Ruiz, M.G.; Pliego, J.A.; Noreña Franco, L.E.; Álvarez, C.M.; Pariente, J.P.; Martin Guaregua, N.C. Synthesis and characterization of a mesoporous cerium oxide catalyst for the conversion of glycerol. J. Appl. Res. Technol. 2018, 16, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Mahfouz, R.; Estephane, J.; Gennequin, C.; Tidahy, L.; Aouad, S.; Abi-Aad, E. CO2 reforming of methane over Ni and/or Ru catalysts supported on mesoporous KIT-6: Effect of promotion with Ce. J. Environ. Chem. Eng. 2021, 9, 104662. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Liu, S.; Ye, D.; Qu, R.; Zheng, C.; Gao, X. Engineering nano-ordered of Ni nanoparticles on KIT-6 for enhanced catalytic hydrogenation of nitrobenzene. Appl. Surf. Sci. 2020, 525, 146382. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Syngas production from dry methane reforming over yttrium-promoted nickel-KIT-6 catalysts. Int. J. Hydrog. Energy 2019, 44, 274–286. [Google Scholar] [CrossRef]
- Koltypin, Y.; Fernandez, A.; Rojas, T.C.; Campora, J.; Palma, P.; Prozorov, R.; Gedanken, A. Encapsulation of Nickel Nanoparticles in Carbon Obtained by the Sonochemical Decomposition of Ni(C8H12)2. Chem. Mater. 1999, 11, 1331–1335. [Google Scholar] [CrossRef]
- Moreau, L.M.; Ha, D.H.; Bealing, C.R.; Zhang, H.; Hennig, R.G.; Robinson, R.D. Unintended Phosphorus Doping of Nickel Nanoparticles during Synthesis with TOP: A Discovery through Structural Analysis. Nano Lett. 2012, 12, 4530–4539. [Google Scholar] [CrossRef]
- Pan, Y.; Jia, R.; Zhao, J.; Liang, J.; Liu, Y.; Liu, C. Size-controlled synthesis of monodisperse nickel nanoparticles and investigation of their magnetic and catalytic properties. Appl. Surf. Sci. 2014, 316, 276–285. [Google Scholar] [CrossRef]
- Jia, F.L.; Zhang, L.Z.; Shang, X.Y.; Yang, Y. Non-Aqueous Sol–Gel Approach towards the Controllable Synthesis of Nickel Nanospheres, Nanowires, and Nanoflowers. Adv. Mater. 2008, 20, 1050–1054. [Google Scholar] [CrossRef]
- Liu, Z.C.; Zhou, J.; Cao, K.; Yang, W.M.; Gao, H.X.; Wang, Y.D.; Li, H.X. Highly dispersed nickel loaded on mesoporous silica: One-spot synthesis strategy and high performance as catalysts for methane reforming with carbon dioxide. Appl. Catal. B 2012, 125, 324–330. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhang, J.; Guo, C.L.; Chen, J.G.; Ren, X.K. Highly dispersed Ni nanoparticles on 3D-mesoporous KIT-6 for CO methanation: Effect of promoter species on catalytic performance. Chin. J. Catal. 2017, 38, 1127–1137. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.V.; Sreedhar, I.; Raghavan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sust. Energ. Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Sanz-Perez, E.S.; Dantas, T.C.M.; Arencibia, A.; Calleja, G.; Guedes, A.P.M.A.; Araujo, A.S.; Sanz, R. Reuse and recycling of amine-functionalized silica materials for CO2 adsorption. Chem. Eng. J. 2017, 308, 1021–1033. [Google Scholar] [CrossRef]
- Muchan, P.; Saiwan., C.C.; Nithitanakul, M. Investigation of adsorption/desorption performance by aminopropyltriethoxysilane grafted onto different mesoporous silica for post-combustion CO2 capture. Clean Energy 2020, 4, 120–131. [Google Scholar] [CrossRef]
- Yılmaz, S.M. A study of CO2 adsorption behaviour and kinetics on KIT-6. EJOSAT 2020, 19, 48–55. [Google Scholar]
- Gibson, J.A.A.; Gromov, A.V.; Brandani, S.; Campbell, E.E.B. The effect of pore structure on the CO2 adsorption efficiency of polyamine impregnated porous carbons. Microporous Mesoporous Mater. 2015, 208, 129–139. [Google Scholar]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoupouros silica: Synthesis and replication to palatinum nanowires, carbon nanorods and carbon nanotube. Chem. Commun. 2003, 7, 2136–2137. [Google Scholar]










| No. | Sample | Specific Surface Area (m2/g) | Pore Volume BJHDes (cc/g) | Average Pore Diameter BJHDes (nm) |
|---|---|---|---|---|
| 1 | KIT-6 | 813 | 0.991 | 7.867 |
| 2 | KIT-6 Sil | 561 | 0.950 | 5.462 |
| 3 | Ni/KIT-6 | 551 | 0.650 | 4.723 |
| 4 | Ni/KIT-6 Sil | 260 | 0.395 | 5.666 |
| 5 | Ce/KIT-6 | 649 | 0.490 | 4.896 |
| No. | Sample | Temp (°C) | nCO2/gSiO2 (mmol/gSiO2) | nCO2/nNH2 (mmol/mmol) |
|---|---|---|---|---|
| 1 | KIT-6-Sil | 40 | 2.23 | 0.51 |
| 2 | KIT-6-Sil | 50 | 1.76 | 0.40 |
| 3 | KIT-6-Sil | 60 | 1.31 | 0.29 |
| 4 | KIT-6-Sil | 70 | 0.95 | 0.22 |
| No. | Sample | Temperature (°C) | nCO2/g SiO2 (mmol/g SiO2) | nCO2/nNH2 (mmol/mmol) |
|---|---|---|---|---|
| 1 | KIT-6 Sil | 40 | 2.23 | 0.512 |
| 2 | Ni/KIT-6 Sil | 40 | 1.63 | 0.188 |
| 3 | Ce/KIT-6 Sil | 40 | 3.66 | 0.256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suba, M.; Popa, A.; Verdeș, O.; Borcănescu, S.; Barvinschi, P. Ni and Ce Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption. Catalysts 2022, 12, 1339. https://doi.org/10.3390/catal12111339
Suba M, Popa A, Verdeș O, Borcănescu S, Barvinschi P. Ni and Ce Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption. Catalysts. 2022; 12(11):1339. https://doi.org/10.3390/catal12111339
Chicago/Turabian StyleSuba, Mariana, Alexandru Popa, Orsina Verdeș, Silvana Borcănescu, and Paul Barvinschi. 2022. "Ni and Ce Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption" Catalysts 12, no. 11: 1339. https://doi.org/10.3390/catal12111339