Surface Modification of GdMn2O5 for Catalytic Oxidation of Benzene via a Mild A-Site Sacrificial Strategy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure
2.2. Morphology and Surface Area
2.3. Surface Composition
2.4. Oxidation-Reduction Property
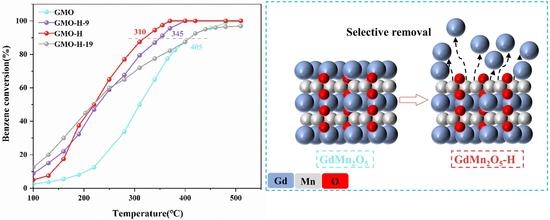
2.5. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Catalyst Characterization
3.4. Evaluation of Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, A.Q.; Gao, S.; Wan, X.; Wang, L.X.; Zhang, T.; Wang, L.; Lang, X.Y.; Wang, W.C. Labile oxygen promotion of the catalytic oxidation of acetone over a robust ternary Mn-based mullite GdMn2O5. Appl. Catal. B-Environ. 2020, 271, 10. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wantz, E.; Kane, A.; Lhuissier, M.; Amrane, A.; Audic, J.L.; Couvert, A. A mathematical model for VOCs removal in a treatment process coupling absorption and biodegradation. Chem. Eng. J. 2021, 423, 8. [Google Scholar] [CrossRef]
- Dong, X.; Tian, Q.; Lu, Y.; Liu, X.; Tong, Y.; Li, K.; Li, Z. Controllable crystallization engineering on amorphous tungsten oxide nanoparticles for highly efficient photochromic response. Sol. Energy Mater. Sol. Cells 2022, 245, 111892. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Chaudary, A.; Mubasher, M.M.; Ul Qounain, S.W. Modeling the Strategies to Control the Impact of Photochemical Smog on Human Health. In Proceedings of the 4th International Conference on Innovative Computing (ICIC), Univ Management & Technol, Lahore, Pakistan, 9–10 November 2021; pp. 36–44. [Google Scholar]
- Javed, A.; Aamir, F.; Gohar, U.F.; Mukhtar, H.; Zia-Ui-Haq, M.; Alotaibi, M.O.; Bin-Jumah, M.N.; Marc, R.A.; Pop, O.L. The Potential Impact of Smog Spell on Humans’ Health Amid COVID-19 Rages. Int. J. Environ. Res. Public Health 2021, 18, 11408. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.; Yang, Z.Q.; Wang, P.; Yan, Y.F.; Ran, J.Y. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 16. [Google Scholar] [CrossRef]
- Yang, C.T.; Miao, G.; Pi, Y.H.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhu, L.L.; Shen, D.K.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 27. [Google Scholar] [CrossRef]
- Wang, H.C.; Guo, H.; Zhao, Y.X.; Dong, X.Q.; Gong, M.Q. Thermodynamic analysis of a petroleum volatile organic compounds (VOCs) condensation recovery system combined with mixed-refrigerant refrigeration. Int. J. Refrig. 2020, 116, 23–35. [Google Scholar] [CrossRef]
- Ozturk, B.; Kuru, C.; Aykac, H.; Kaya, S. VOC separation using immobilized liquid membranes impregnated with oils. Sep. Purif. Technol. 2015, 153, 1–6. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W.; Cyganiuk, A.; Dumee, L.F.; Al-Gharabli, S. Upgrading of zirconia membrane performance in removal of hazardous VOCs from water by surface functionalization. Chem. Eng. J. 2019, 374, 155–169. [Google Scholar] [CrossRef]
- Yang, P.; Fan, S.K.; Chen, Z.Y.; Bao, G.F.; Zuo, S.F.; Qi, C.Z. Synthesis of Nb2O5 based solid superacid materials for catalytic combustion of chlorinated VOCs. Appl. Catal. B-Environ. 2018, 239, 114–124. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, B.J.; Li, Z.J.; Zhang, X.J.; Liu, R.J.; Yun, J. Amorphous-microcrystal combined manganese oxides for efficiently catalytic combustion of VOCs. Mol. Catal. 2020, 489, 8. [Google Scholar] [CrossRef]
- Meena, M.; Sonigra, P.; Yadav, G. Biological-based methods for the removal of volatile organic compounds (VOCs) and heavy metals. Environ. Sci. Pollut. Res. 2021, 28, 2485–2508. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean Prod. 2021, 278, 23. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranova, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Skapin, A.S.; Calvino, J.J.; Brezova, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405, 14. [Google Scholar] [CrossRef]
- Guo, Y.L.; Wen, M.C.; Li, G.Y.; An, T.C. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B-Environ. 2021, 281, 19. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Mao, J.X.; Zhou, R.X. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature. Appl. Surf. Sci. 2019, 465, 15–22. [Google Scholar] [CrossRef]
- Guo, Y.L.; Gao, Y.J.; Li, X.; Zhuang, G.L.; Wang, K.C.; Zheng, Y.; Sun, D.H.; Huang, J.L.; Li, Q.B. Catalytic benzene oxidation by biogenic Pd nanoparticles over 3D-ordered mesoporous CeO2. Chem. Eng. J. 2019, 362, 41–52. [Google Scholar] [CrossRef]
- Liu, Y.X.; Dai, H.X.; Deng, J.G.; Xie, S.H.; Yang, H.G.; Tan, W.; Han, W.; Jiang, Y.; Guo, G.S. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.X.; Deng, J.G.; Zhao, X.T.; Yang, J.; Han, Z.; Hou, Z.Q.; Dai, H.X. Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: Highly active catalysts for benzene combustion. Appl. Catal. B-Environ. 2019, 244, 650–659. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Li, L.; Peng, Y.; Wang, D.; Ma, Y.; Li, J. Synthesis of alpha-MnO2-like rod catalyst using YMn2O5 A-site sacrificial strategy for efficient benzene oxidation. J. Hazard. Mater. 2021, 403, 123811. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.P.; Li, S.D.; Li, W.H.; Li, J.Q.; Chen, J.Y.; Chen, Y.F. Excellent low temperature performance for total benzene oxidation over mesoporous CoMnAl composited oxides from hydrotalcites. J. Mater. Chem. A 2016, 4, 8113–8122. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zuo, J.C.; Luo, Y.J.; Jiang, L.L. New route to CeO2/LaCoO3 with high oxygen mobility for total benzene oxidation. Appl. Surf. Sci. 2017, 396, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Liu, Y.; Zhang, T.H.; Luo, Y.J.; Lan, Z.X.; Zhang, K.; Zuo, J.C.; Jiang, L.L.; Wang, R.H. Geometrical-Site-Dependent Catalytic Activity of Ordered Mesoporous Co-Based Spinel for Benzene Oxidation: In Situ DRIFTS Study Coupled with Raman and XAFS Spectroscopy. ACS Catal. 2017, 7, 1626–1636. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Wang, X.; Li, Q.; Li, L.; Yang, W.; Chu, X.; Liu, H.; Men, J.; Peng, Y.; et al. Surface Reconstruction of a Mullite-Type Catalyst via Selective Dissolution for NO Oxidation. ACS Catal. 2021, 11, 14507–14520. [Google Scholar] [CrossRef]
- Liu, R.Y.; Zhou, B.; Liu, L.Z.; Zhang, Y.; Chen, Y.; Zhang, Q.L.; Yang, M.L.; Hu, L.P.; Wang, M.; Tang, Y.F. Enhanced catalytic oxidation of VOCs over porous Mn-based mullite synthesized by in-situ dismutation. J. Colloid Interface Sci. 2021, 585, 302–311. [Google Scholar] [CrossRef]
- Guo, M.; Li, K.; Zhang, H.; Min, X.; Hu, X.; Guo, W.; Jia, J.; Sun, T. Enhanced catalytic activity of oxygenated VOC deep oxidation on highly active in-situ generated GdMn2O5/GdMnO3 catalysts. J. Colloid Interface Sci. 2020, 578, 229–241. [Google Scholar] [CrossRef]
- Khatun, N.; Rini, E.G.; Shirage, P.; Rajput, P.; Jha, S.N.; Sen, S. Effect of lattice distortion on bandgap decrement due to vanadium substitution in TiO2 nanoparticles. Mater. Sci. Semicond. Process 2016, 50, 7–13. [Google Scholar] [CrossRef]
- Li, S.D.; Zhao, G.X.; Bi, H.; Huang, Z.G.; Lai, H.; Gai, R.Q.; Du, Y.W. Synthesis and anomalous magnetic properties of CoCr2O4 nanocrystallites with lattice distortion. J. Magn. Magn. Mater. 2006, 305, 448–451. [Google Scholar] [CrossRef]
- Lin, T.G.; Wang, L.P.; Wang, X.F.; Zhang, Y.F.; Yu, Y.H. Influence of lattice distortion on phase transition properties of polycrystalline VO2 thin film. Appl. Surf. Sci. 2016, 379, 179–185. [Google Scholar] [CrossRef]
- O’Connell, J.H.; Lee, M.E.; Yagoub, M.Y.A.; Swart, H.C.; Coetsee, E. Characterization of crystallite morphology for doped strontium fluoride nanophosphors by TEM and XRD. Phys. B 2016, 480, 169–173. [Google Scholar] [CrossRef]
- Zhua, G.; Liu, P.; Hojamberdiev, M.; Ge, B.; Liu, Y.; Miao, H.; Tan, G. Synthesis RMn2O5 (R = Gd and Sm) nano- and microstructures by a simple hydrothermal method. Mater. Chem. Phys. 2009, 118, 467–472. [Google Scholar] [CrossRef]
- Gorzalski, A.S.; Donley, C.; Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 2017, 522, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Chaikittisilp, W.; Koike, N.; Yokoi, T.; Okubo, T. Porous inorganic-organic hybrid polymers derived from cyclic siloxane building blocks: Effects of substituting groups on mesoporous structures. Microporous Mesoporous Mater. 2019, 278, 212–218. [Google Scholar] [CrossRef]
- Chen, J.H.; Shen, M.Q.; Wang, X.Q.; Qi, G.S.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B-Environ. 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lu, Y.; Song, C.J.; Ma, Z.C.; Xing, S.T.; Gao, Y.Z. A novel redox-precipitation method for the preparation of alpha-MnO2 with a high surface Mn4+ concentration and its activity toward complete catalytic oxidation of o-xylene. Catal. Today 2013, 201, 32–39. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.Y.; Fang, Y.R.; Hoang, S.; Li, L.; Yang, W.W.; Liang, Z.F.; Wu, J.; Hu, J.P.; Xiao, W.; et al. Oxygen Vacancy Promoted O-2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Mo, S.P.; Zhang, Q.; Li, J.Q.; Sun, Y.H.; Ren, Q.M.; Zou, S.B.; Zhang, Q.; Lu, J.H.; Fu, M.L.; Mo, D.Q.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B-Environ. 2020, 264, 16. [Google Scholar] [CrossRef]
- Wu, M.D.; Chen, S.Y.; Xiang, W.G. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chem. Eng. J. 2020, 387, 12. [Google Scholar] [CrossRef]







| Catalyst | ICP (ppm) | Atomic% (TEM-EDS Mapping) | Surface Element Molar Ratio (XPS) | |||
|---|---|---|---|---|---|---|
| Gd/Mn | Gd/Mn | Gd/Mn | Olatt/Oads | Mn4+/Mn3+ | AOS | |
| GMO | 0.47 | 0.46 | 0.73 | 4.27 | 0.31 | 3.1 |
| GMO-H | 0.39 | 0.40 | 0.33 | 3.48 | 0.42 | 3.3 |
| Catalyst | Surface Area (m2 g−1) | Pore Size(nm) | Ea (kJ mol−1) | H2 Consumption (mmol g−1) | O2-TPD Peak Area | Benzene Conversions a (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | α | β | T50% | T90% | T100% | |||||
| GMO | 30.4 | 16.4 | 32.5 | 3.74 | 0.50 | 0.16 | 0.34 | 310 | 405 | 490 |
| GMO-H-9 | 48.6 | 11.7 | 24.7 | 235 | 345 | 395 | ||||
| GMO-H | 52.9 | 10.9 | 23.4 | 4.20 | 626.02 | 221.22 | 404.80 | 220 | 310 | 340 |
| GMO-H-19 | 46.5 | 11.4 | 32.5 | 225 | 405 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Dong, X.; Li, Z.; Shao, Y.; Ren, X.; Li, K.; Lu, Y. Surface Modification of GdMn2O5 for Catalytic Oxidation of Benzene via a Mild A-Site Sacrificial Strategy. Catalysts 2022, 12, 1267. https://doi.org/10.3390/catal12101267
Gao J, Dong X, Li Z, Shao Y, Ren X, Li K, Lu Y. Surface Modification of GdMn2O5 for Catalytic Oxidation of Benzene via a Mild A-Site Sacrificial Strategy. Catalysts. 2022; 12(10):1267. https://doi.org/10.3390/catal12101267
Chicago/Turabian StyleGao, Ju, Xu Dong, Zhenguo Li, Yuankai Shao, Xiaoning Ren, Kaixiang Li, and Yiren Lu. 2022. "Surface Modification of GdMn2O5 for Catalytic Oxidation of Benzene via a Mild A-Site Sacrificial Strategy" Catalysts 12, no. 10: 1267. https://doi.org/10.3390/catal12101267