Zinc–Acetate–Amine Complexes as Precursors to ZnO and the Effect of the Amine on Nanoparticle Morphology, Size, and Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
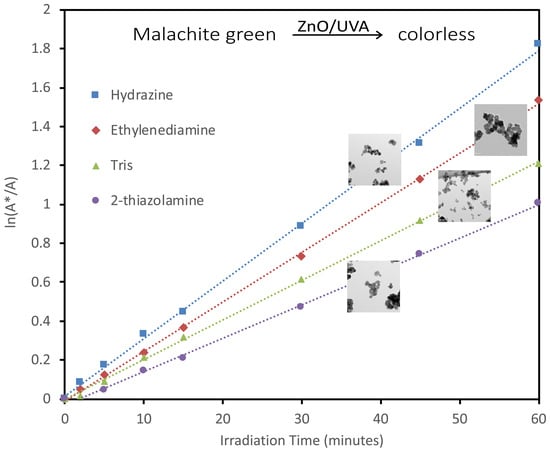
2.2. Photocatalytic Activity
2.3. Catalyst Reusability
3. Materials and Methods
3.1. Techniques and Materials
3.2. ZnO Precursor Isolation and Characterization
3.3. ZnO Particle Synthesis
3.4. Powder X-ray Diffraction
3.5. Scanning Electron Microscopy
3.6. Transmission Electron Microscopy
3.7. Ultraviolet–Visible Diffuse Reflectance Spectroscopy
3.8. Surface Area Measurements
3.9. Thermal Analysis
3.10. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janotti, A.; van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morko, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X. Enhanced fluorescence imaging performance of hydrophobic colloidal ZnO nanoparticles by a facile method. J. Alloys Compd. 2015, 619, 98–101. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Babitha, N.; Priya, L.S.; Christy, S.R.; Manikandan, A.; Dinesh, A.; Durka, M.; Arunadevi, S. Enhanced Antibacterial Activity and Photo-Catalytic Properties of ZnO Nanoparticles: Pedalium Murex Plant Extract-Assisted Synthesis. J. Nanosci. Nanotechnol. 2019, 19, 2888–2894. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z. Synthesis of nanowires, nanorods and nanoparticles of ZnO through modulating the ratio of water to methanol by using a mild and simple solution method. Mater. Chem. Phys. 2005, 89, 326–331. [Google Scholar] [CrossRef]
- Bhushan, B.; Murty, B.; Mondal, K. A new approach for synthesis of ZnO nanorod flowerets and subsequent pure free-standing ZnO nanorods. Adv. Powder Technol. 2019, 30, 30–41. [Google Scholar] [CrossRef]
- Cossuet, T.; Roussel, H.; Chauveau, J.-M.; Chaix-Pluchery, O.; Thomassin, J.-L.; Appert, E.; Consonni, V. Well-ordered ZnO nanowires with controllable inclination on semipolar ZnO surfaces by chemical bath deposition. Nanotechnology 2018, 29, 475601. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Direct growth of enclosed ZnO nanotubes. Nano Res. 2009, 2, 201–209. [Google Scholar] [CrossRef]
- Choi, K.-S.; Chang, S.-P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Mina, S.-K.; Manea, R.S.; Joob, O.-S.; Ganesha, T.; Choc, B.W.; Han, S.-H. Upright-standing ZnO nano-sheets growth using wet chemistry. Curr. Appl. Phys. 2009, 9, 492–495. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.-S. Hydro/solvo-thermal synthesis of ZnO crystallite with particular morphology. Trans. Nonferrous Metal Soc. China 2009, 19, 399–403. [Google Scholar] [CrossRef]
- Li, P.; Zhu, S.; Hu, H.; Guo, L.; He, T. Influence of defects in porous ZnO nanoplates on CO2 photoreduction. Catal. Today 2019, 335, 300–305. [Google Scholar] [CrossRef]
- Zhang, L.; Jeem, M.; Okamoto, K.; Watanabe, S. Photochemistry and the role of light during the submerged photosynthesis of zinc oxide nanorods. Sci. Rep. 2018, 8, 177. [Google Scholar] [CrossRef]
- Xu, Z.; Ben, Y.; Chen, Z.; Qi, F. Facile synthesis of snowflake-like ZnO nanostructures at low temperature and their super catalytic activity for the ozone decomposition. Mater. Res. Bull. 2013, 48, 1725–1727. [Google Scholar] [CrossRef]
- Sigoli, F.A.; Davolos, M.R.; Jafelicci, M., Jr. Morphological evolution of zinc oxide originating from zinc hydroxide carbonate. J. Alloys Compd. 1997, 262–263, 292–295. [Google Scholar] [CrossRef]
- Wang, L.; Muhammed, M. Synthesis of zinc oxide nanoparticles with controlled morphology. J. Mater. Chem. 1999, 9, 2871–2878. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, G. Synthesis and characterization of ZnO nanorods. J. Cryst. Growth 2003, 252, 213–218. [Google Scholar] [CrossRef]
- Meagley, K.L.; Garcia, S.P. Chemical Control of Crystal Growth with Multidentate Carboxylate Ligands: Effect of Ligand Denticity on Zinc Oxide Crystal Shape. Cryst. Growth Des. 2012, 12, 707–713. [Google Scholar] [CrossRef]
- Anders, C.B.; Eixenberger, J.E.; Franco, N.A.; Hermann, R.J.; Rainey, K.D.; Chess, J.J.; Punnoose, A.; Wingett, D.G. ZnO nano-particle preparation route influences surface reactivity, dissolution and cytotoxicity. Environ. Sci. Nano 2018, 5, 572–588. [Google Scholar] [CrossRef]
- Marin, O.; González, V.; Tirado, M.; Comedi, D. Effects of methanol on morphology and photoluminescence in solvothermal grown ZnO powders and ZnO on Si. Mater. Lett. 2019, 251, 41–44. [Google Scholar] [CrossRef]
- Andrés-Vergés, M.; Martínez-Gallego, M. Spherical and rod-like zinc oxide microcrystals: Morphological characterization and microstructural evolution with temperature. J. Mater. Sci. 1992, 27, 3756–3762. [Google Scholar] [CrossRef]
- Noack, V.; Eychmüller, A. Annealing of Nanometer-Sized Zinc Oxide Particles. Chem. Mater. 2002, 14, 1411–1417. [Google Scholar] [CrossRef]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-Gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Films 1997, 306, 78–85. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Zhao, B.; Bao, X. Size control of ZnO nanoparticles via thermal decomposition of zinc acetate coated on organic additives. J. Cryst. Growth 2004, 263, 447–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Xu, H.; Chin, W.-S. Shape-Controlled Synthesis of Zinc Oxide: A Simple Method for the Preparation of Metal Oxide Nanocrystals in Non-aqueous Medium. Chem. Eur. J. 2006, 13, 632–638. [Google Scholar] [CrossRef]
- Bacaksiz, E.; Parlak, M.; Tomakin, M.; Özçelik, A.; Karakız, M.; Altunbaş, M. The effects of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloy. Compd. 2008, 466, 447–450. [Google Scholar] [CrossRef]
- Ebrahimifard, R.; Abdizadeh, H.; Golobostanfard, M.R. Controlling the extremely preferred orientation texturing of sol–gel derived ZnO thin films with sol and heat treatment parameters. J. Sol-Gel Sci. Technol. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Ravichandran, K.; Begum, N.J.; Snega, S.; Sakthivel, B. Properties of Sprayed Aluminum-Doped Zinc Oxide Films—A Review. Mater. Manuf. Process. 2016, 31, 1411–1423. [Google Scholar] [CrossRef]
- Eensalu, J.S.; Krunks, M.; Gromyko, I.; Katerski, A.; Mere, A. A comparative study on physical properties of Al-doped zinc oxide thin films deposited from zinc acetate and zinc acetylacetonate by spray pyrolysis. Energetika 2017, 63, 46–55. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.-S.; Pode, R.B. Low temperature dielectric studies of zinc oxide (ZnO) na-noparticles prepared by precipitation method. Adv. Powder Technol. 2013, 24, 331–335. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Liu, H.; Wang, X.-K. Growth of well-defined ZnO microparticles with additives from aqueous solution. J. Solid State Chem. 2005, 178, 855–860. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, L.; Tanemura, S.; Tanemura, M.; Kuno, Y.; Hayashi, Y. Low resistivity p-ZnO films fabricated by sol-gel spin coating. Appl. Phys. Lett. 2006, 88, 251116. [Google Scholar] [CrossRef]
- Kittilstved, K.R.; Norberg, N.S.; Gamelin, D.R. Chemical manipulation of high-TC ferromagnetism in ZnO diluted magnetic semiconductors. Phys. Rev. Lett. 2005, 94, 147209. [Google Scholar] [CrossRef]
- Vajargah, P.H.; Abdizadeh, H.; Ebrahimifard, R.; Golobostanfard, M.R. Sol-gel derived ZnO thin films: Effect of ami-no-additives. Appl. Surf. Sci. B 2013, 285, 732–743. [Google Scholar] [CrossRef]
- Hyslop, J.S.; Boydstun, A.R.; Fereday, T.R.; Rusch, J.R.; Strunk, J.L.; Wall, C.T.; Pena, C.C.; McKibben, N.L.; Harris, J.D.; Thurber, A.; et al. Synthesis and characterization of [Zn(acetate)2(amine)] compounds (x = 1 or 2) and their use as precursors to ZnO. Mater. Sci. Semicond. Process. 2015, 38, 278–289. [Google Scholar] [CrossRef]
- Culp, S.J.; Beland, F.A. Malachite Green: A Toxicological Review. J. Am. Coll. Toxicol. 1996, 15, 219–238. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Andrade, J.D.L.; Montanha, M.C.; Lima, S.M.; Andrade, L.H.D.C.; Hechenleitner, A.A.W.; Pineda, E.A.G.; de Oliveira, D.M.F. Decontamination and disinfection of wastewater by photocatalysis under UV/visible light using nano-catalysts based on Ca-doped ZnO. J. Environ. Manag. 2019, 240, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.-K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef] [PubMed]
- Tayyebi, A.; Outokesh, M.; Tayebi, M.; Shafikhani, A.; Şengör, S.S. ZnO quantum dots-graphene composites: Formation mecha-nism and enhanced photocatalytic activity for degradation of methyl orange dye. J. Alloys Compd. 2016, 663, 738–749. [Google Scholar] [CrossRef]
- Tayyebi, A.; Soltani, T.; Lee, B.-K.; Outokesh, M.; Tayebi, M. Novel Visible Light Photocatalytic and Photoelectrochemical (PEC) Activity of Carbon-doped Zinc Oxide/Reduced Graphene Oxide: Supercritical Methanol Synthesis with Enhanced Photocor-rosion Suppression. J. Alloys Compd. 2017, 723, 1001–1010. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets. Appl. Surf. Sci. 2020, 502, 144189. [Google Scholar] [CrossRef]
- Elias; Uddin, N.; Saha, J.; Hossain, A.; Sarker, D.; Akter, S.; Siddiquey, I.; Uddin, J. A Highly Efficient and Stable Photocatalyst; N-Doped ZnO/CNT Composite Thin Film Synthesized via Simple Sol-Gel Drop Coating Method. Molecules 2021, 26, 1470. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, Y. Photocatalytic degradation for methylene blue using zinc oxide prepared by codeposition and sol-gel methods. J. Hazard. Mater. 2008, 152, 172–175. [Google Scholar] [CrossRef]
- Shifu, C.; Wei, Z.; Sujuan, Z.; Wei, L. Preparation, characterization and photocatalytic activity of N-containing ZnO powder. Chem. Eng. J. 2009, 148, 263–269. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhu, L.; He, H.; Hu, L.; Huang, J.; Hu, F.; He, B.; Ye, Z. Shape control of colloidal Mn doped ZnO nanocrystals and their visible light photocatalytic properties. Nanoscale 2013, 5, 10461–10471. [Google Scholar] [CrossRef] [PubMed]
- Giahi, M.; Farajpour, G.; Taghavi, H.; Shokri, S. Preparation of Photocatalytic ZnO Nanoparticles and Application in Photo-chemical Degradation of Betamethasone Sodium Phosphate Using Taguchi Approach. Russ. J. Phys. Chem. A 2014, 88, 1241–1247. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Ghiloufi, I.; Mir, L.E. Adsorption and photocatalytic degradation of malachite green by vanadium doped zinc oxide nanoparticles. Water Sci. Technol. 2015, 73, 881–889. [Google Scholar] [CrossRef]
- Meena, S.; Vaya, D.; Das, B.K. Photocatalytic degradation of Malachite Green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 2016, 39, 1735–1743. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K. Photocatalytic degradation of Reactive Black 5 and Malachite Green with ZnO and lanthanum doped nanoparticles. J. Phys. Conf. Ser. 2016, 682, 12022. [Google Scholar] [CrossRef]
- Horzum, N.; Hilal, M.E.; Isık, T. Enhanced bactericidal and photocatalytic activities of ZnO nanostructures by changing the cooling route. New J. Chem. 2018, 42, 11831–11838. [Google Scholar] [CrossRef]
- Babajani, N.; Jamshidi, S. Investigation of photocatalytic malachite green degradation by iridium doped zinc oxide nanoparti-cles: Application of response surface methodology. J. Alloys Compd. 2019, 782, 533–544. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abukhadra, M.R.; Rady, A.M.; Ahmed, S.A.; Labena, A.; Mohamed, H.S.H.; Betiha, M.A.; Shim, J.-J. Instantaneous photocatalytic degradation of malachite green dye under visible light using novel green Co–ZnO/algae composites. Res. Chem. Intermed. 2020, 46, 1955–1973. [Google Scholar] [CrossRef]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; de Stefano, L.; Aronne, A.; Vaiano, V. F-doped ZnO nano- and meso-crystals with enhanced photocatalytic activity in diclofenac degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.; McDonagh, A. Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide. Dalton Trans. 2016, 45, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D. Optimization of process parameters and its effect on particle size and morphology of ZnO nanoparticle synthesized by sol–gel method. J. Sol-Gel Sci. Technol. 2016, 77, 48–56. [Google Scholar] [CrossRef]
- Harun, K.; Hussain, F.; Purwanto, A.; Sahraoui, B.; Zawadzka, A.; Mohamad, A.A. Sol–gel synthesized ZnO for optoelectronics applications: A characterization review. Mater. Res. Express 2017, 4, 122001. [Google Scholar] [CrossRef]
- Spanhel, L. Colloidal ZnO nanostructures and functional coatings: A survey. J. Sol-Gel Sci. Technol. 2006, 39, 7–24. [Google Scholar] [CrossRef]
- Briois, V.; Giorgetti, C.; Baudelet, F.; Blanchandin, S.; Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V. Dynamical Study of ZnO Nanocrystal and Zn-HDS Layered Basic Zinc Acetate Formation from Sol−Gel Route. J. Phys. Chem. C 2007, 111, 3253–3258. [Google Scholar] [CrossRef]
- Zheng, Z.; Butynska, R.; Serrano, C.V.; Marty, J.-D.; Mingotaud, C.; Kahn, M.L. One-Step Synthesis of Hybrid Liquid-Crystal ZnO Nanoparticles: Existence of a Critical Temperature Associated with the Anisotropy of the Nanoparticles. Chem. Eur. J. 2016, 22, 15614–15618. [Google Scholar] [CrossRef]
- Jia, G.; Xu, S.; Wang, A. Emerging strategies for the synthesis of monodisperse colloidal semiconductor quantum rods. J. Mater. Chem. C 2015, 3, 8284–8293. [Google Scholar] [CrossRef]
- Herrera-Rivera, R.; de la Olvera, M.L.; Maldonado, A. Synthesis of ZnO Nanopowders by the Homogeneous Precipitation Method: Use of Taguchi’s Method for Analyzing the Effect of Different Variables. J. Nanomater. 2017, 2017, 4595384. [Google Scholar] [CrossRef]
- Alnarabiji, M.S.; Yahya, N.; Hamed, Y.; Ardakani, S.E.M.; Azizi, K.; Klemeš, J.J.; Abdullah, B.; Tasfy, S.F.H.; Hamid, S.B.A.; Nashed, O. Scalable bio-friendly method for production of homogeneous metal oxide nanoparticles using green bovine skin gel-atin. J. Cleaner Prod. 2017, 162, 186–194. [Google Scholar] [CrossRef]
- Li, J.-G.; Ikegami, T.; Wang, Y.; Mori, T. 10-mol%-Gd2O3-Doped CeO2 Solid Solutions via Carbonate Coprecipitation: A Com-parative Study. J. Am. Ceram. Soc. 2003, 86, 915–921. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Chemiresistive Sensor Based on Zinc Oxide Nanoflakes for CO2 Detection. ACS Appl. Nano Mater. 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Quy, C.T.; Thai, N.X.; Hoa, N.D.; Le, D.T.T.; Hung, C.M.; Duy, N.V.; Hieu, N.V. C2H5OH and NO2 sensing properties of ZnO nanostructures: Correlation between crystal size, defect level and sensing performance. RSC Adv. 2018, 8, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Shaporev, A.S.; Ivanov, V.K.; Baranchikov, A.E.; Polezhaeva, O.S.; Tret’Yakov, Y.D. ZnO formation under hydrothermal conditions from zinc hydroxide compounds with various chemical histories. Russ. J. Inorg. Chem. 2007, 52, 1811–1816. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fís. S 2007, 53, 18–22. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447. [Google Scholar] [CrossRef]
- Ramelan, A.H.; Wahyuningsih, S.; Munawaroh, H.; Narayan, R. ZnO wide bandgap semiconductors preparation for optoe-lectronic devices. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012008. [Google Scholar] [CrossRef]
- Eixenberger, J.E.; Anders, C.B.; Wada, K.; Reddy, K.M.; Brown, R.J.; Moreno-Ramirez, J.; Weltner, A.E.; Karthik, C.; Tenne, D.A.; Fologea, D.; et al. Defect Engineering of ZnO Nanoparticles for Bioimaging Applications. ACS Appl. Mater. Interfaces 2019, 11, 24933–24944. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solu-tion. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Rabieh, S.; Bagheri, M.; Heydari, M.; Badiei, E. Microwave assisted synthesis of ZnO nanoparticles in ionic liquid [Bmim]cl and their photocatalytic investigation. Mater. Sci. Semicond. Process. 2014, 26, 244–250. [Google Scholar] [CrossRef]
- Prasad, K.S.; Prajapati, S.; Selvaraj, K. Efficient sorption and photocatalytic degradation of malachite green dye onto NiS na-noparticles prepared using novel green approach. Korean J. Chem. Eng. 2015, 32, 1986–1992. [Google Scholar] [CrossRef]
- Jo, W.-K.; Park, G.T.; Tayade, R.J. Synergetic effect of adsorption on degradation of malachite green dye under blue LED irra-diation using spiral-shaped photocatalytic reactor. J. Chem. Technol. Biotechnol. 2015, 90, 2280–2289. [Google Scholar] [CrossRef]
- Goswami, T.; Reddy, K.M.; Bheemaraju, A. Silver Nanocluster Anchored TiO2/Nb2O5 Hybrid Nanocomposite as Highly Efficient and Selective Visible-Light Sensitive Photocatalyst. ChemistrySelect 2019, 4, 6790–6799. [Google Scholar] [CrossRef]
- Nethaji, S.; Tamilarasan, G.; Neehar, P.; Sivasamy, A. Visible light photocatalytic activities of BiOBr-activated carbon (derived from waste polyurethane) composites by hydrothermal process. J. Environ. Chem. Eng. 2018, 6, 3735–3744. [Google Scholar]
- Ghaedi, M.; Ansari, A.; Habibi, M.H.; Asghari, A.R. Removal of malachite green from aqueous solution by zinc oxide nanopar-ticle loaded on activated carbon: Kinetics and isotherm study. J. Ind. Eng. Chem. 2014, 20, 17–28. [Google Scholar] [CrossRef]
- Pare, B.; Sarwan, B.; Jonnalagadda, S.B. Photocatalytic mineralization study of malachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition. Appl. Surf. Sci. 2011, 258, 247–253. [Google Scholar] [CrossRef]
- Liu, Y.; Ohko, Y.; Zhang, R.; Yang, Y.; Zhang, Z. Degradation of malachite green on Pd/WO3 photocatalysts under simulated solar light. J. Hazard. Mater. 2010, 184, 386–391. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-Coated Zinc Oxide Nanoparticles as Innovative ROS-Generators for Photodynamic Therapy in Cancer Cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef]
- Yong, L.; Zhanqi, G.; Yuefei, J.; Xiaobin, H.; Cheng, S.; Shaogui, Y.; Lianhong, W.; Qingeng, W.; Die, F. Photodegradation of mal-achite green under simulated and natural irradiation: Kinetics, products, and pathways. J. Hazard. Mater. 2015, 285, 127–136. [Google Scholar] [CrossRef]
- Hepp, A.F.; Bailey, S.G.; McNatt, J.S.; Chandrashekhar, M.V.S.; Harris, J.D.; Rusch, A.W.; Nogales, K.A.; Goettsche, K.V.; Hanson, W.; Amos, D.; et al. Novel Materials, Processing and Device Technologies for Space Exploration with Potential Dual-Use Applications. In NASA Technical Memorandum; NASA/TM—2015-218866; National Aeronautics and Space Administration: Brook park, OH, USA, 2015. [Google Scholar]
- Wang, H.; Xie, C.; Zhang, W.; Cai, S.; Yang, Z.; Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J. Hazard. Mater. 2007, 141, 645–652. [Google Scholar] [CrossRef]
- Sharma, S.; Mehta, S.K.; Kansal, S.K. N doped ZnO/C-dots nanoflowers as visible light driven photocatalyst for the degradation of malachite green dye in aqueous phase. J. Alloys Compd. 2017, 699, 323–333. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Team R. Core. R: A Language and Environment for Statistical Computing, The R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: http://www.R-project.org/.
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Fraté, H.; Ludwig, J.; Kschischo, M. grofit: Fitting Biological Growth Curves with R. J. Stat. Softw. 2010, 33, 1–21. [Google Scholar] [CrossRef] [Green Version]









| Amine | Particle Size (nm) (TEM) | Particle Size (nm) (Scherrer) | Particle Size (nm) (Surface Area) | Surface Area (m2/g) |
|---|---|---|---|---|
| Tris | 20(4) | 15(1) | 24.5 | 43.7 |
| Hydrazine | 27(7) | 17(4) | 30.0 | 35.6 |
| 2-Thiazolamine | 34(9) | 19(1) | 30.5 | 35.1 |
| Ethylenediamine | 45(7) | 18(4) | 66.4 | 16.1 |
| Precursor | Dye Conc. (g/L) | Catalyst Conc. (g/L) | kobs (min−1) | Ref |
|---|---|---|---|---|
| ZnCl2 | 0.01 | 0.2 | 0.010 | [58] |
| Zn(acetate)2 | 0.2 | 0.2 | 0.014 | [53] |
| Zn(acetate)2 + NH3 | 0.02 | 0.05 | 0.009 | [94] |
| Zn(acetate)2 + TEA | 0.01 | 0.12 | 0.0046 | [56] |
| Zn(NO3)2 + PEG | 0.2 | 0.2 | 0.023 | [53] |
| Zn(NO3)2 + EDTA | .0093 | 0.2 | 0.058 | [55] |
| [Zn(acetate)2(hydrazine)2] | 0.027 | 1.67 | 0.038 | this work |
| [Zn(acetate)2(ethylenediamine)] | 0.027 | 1.67 | 0.024 | this work |
| [Zn(acetate)2(Tris)2] | 0.027 | 1.67 | 0.021 | this work |
| [Zn(acetate)2(2-thiazolamine)2] | 0.027 | 1.67 | 0.019 | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, J.D.; Wade, E.A.; Ellison, E.G.; Pena, C.C.; Bryant, S.C.; McKibben, N.L.; Christy, A.J.; Laughlin, K.O.; Harris, A.E.; Goettsche, K.V.; et al. Zinc–Acetate–Amine Complexes as Precursors to ZnO and the Effect of the Amine on Nanoparticle Morphology, Size, and Photocatalytic Activity. Catalysts 2022, 12, 1099. https://doi.org/10.3390/catal12101099
Harris JD, Wade EA, Ellison EG, Pena CC, Bryant SC, McKibben NL, Christy AJ, Laughlin KO, Harris AE, Goettsche KV, et al. Zinc–Acetate–Amine Complexes as Precursors to ZnO and the Effect of the Amine on Nanoparticle Morphology, Size, and Photocatalytic Activity. Catalysts. 2022; 12(10):1099. https://doi.org/10.3390/catal12101099
Chicago/Turabian StyleHarris, Jerry D., Emily A. Wade, Emmaline G. Ellison, Cecelia C. Pena, Stephen C. Bryant, Nicholas L. McKibben, Allison J. Christy, Kevin O. Laughlin, Ashley E. Harris, Kenrik V. Goettsche, and et al. 2022. "Zinc–Acetate–Amine Complexes as Precursors to ZnO and the Effect of the Amine on Nanoparticle Morphology, Size, and Photocatalytic Activity" Catalysts 12, no. 10: 1099. https://doi.org/10.3390/catal12101099