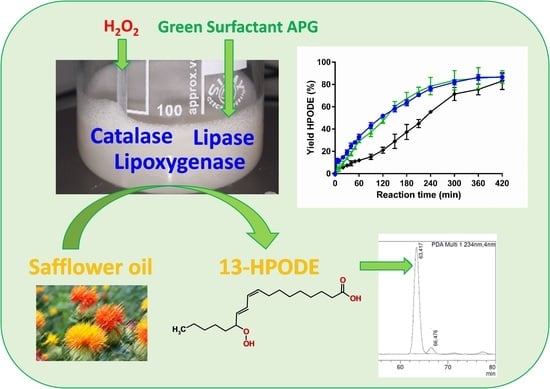
Synthesis of Linoleic Acid 13-Hydroperoxides from Safflower Oil Utilizing Lipoxygenase in a Coupled Enzyme System with In-Situ Oxygen Generation
Abstract
:1. Introduction
2. Results & Discussion
2.1. LOX Preparation and Initial Optimization of LOX Reaction Conditions
2.2. Lipase Selection and Evaluation of Bienzymatic Lipase-LOX-1 Reaction System
2.3. Implementation of an Enzyme Cascade with LOX-1, Lipase and Catalase
3. Materials and Methods
3.1. Materials
3.2. Preparation of Linoleic Acid and Soybean Lipoxygenase
3.3. Enzyme Activity Measurements and Protein Quantification
3.4. Biocatalytic Transformations
3.5. Product Analysis and Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hansen, J.; Garreta, A.; Benincasa, M.; Fusté, M.C.; Busquets, M.; Manresa, A. Bacterial lipoxygenases, a new subfamily of enzymes? A phylogenetic approach. Appl. Microbiol. Biotechnol. 2013, 97, 4737–4747. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Lipoxygenases: Occurrence, Functions, Catalysis, and Acquisition of Substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, A.; Feussner, I. Lipoxygenases Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. Thelipoxygenasepathway. Annu. Rev. Plant. Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Blée, E. Impact of phyto-oxylipins in plant defense. Trends Plant. Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; De Caraffa, V.B.-B.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Yadav, J. Lipoxygenase biocatalysis: A survey of asymmetric oxygenation. J. Mol. Catal. B Enzym. 2003, 26, 3–28. [Google Scholar] [CrossRef]
- Heshof, R.; De Graaff, L.H.; Villaverde, J.J.; Silvestre, A.; Haarmann, T.; Dalsgaard, T.K.; Buchert, J. Industrial potential of lipoxygenases. Crit. Rev. Biotechnol. 2015, 36, 665–674. [Google Scholar] [CrossRef]
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From Isolation to Application. Compr. Rev. Food Sci. Food Saf. 2016, 16, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schörken, U.; Kempers, P. Lipid biotechnology: Industrially relevant production processes. Eur. J. Lipid Sci. Technol. 2009, 111, 627–645. [Google Scholar] [CrossRef]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Gardner, H.W.; Plattner, R.D. Linoleate hydroperoxides are cleaved heterolytically into aldehydes by a Lewis acid in aprotic solvent. Lipids 1984, 19, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kerler, J.; Kohlen, E.; Fitz, W.; Vliet, A.V.D.; Winkel, C. Method for the Enzymatic Preparation of Aldehydes Rich Aromas c6-c10. U.S. Patent No. 6864072, 8 March 2005. [Google Scholar]
- Otte, K.B.; Kirtz, M.; Nestl, B.M.; Hauer, B. Synthesis of 9-Oxononanoic Acid, a Precursor for Biopolymers. ChemSusChem 2013, 6, 2149–2156. [Google Scholar] [CrossRef]
- Löwe, J.; Dietz, K.; Gröger, H. From a Biosynthetic Pathway toward a Biocatalytic Process and Chemocatalytic Modifications: Three-Step Enzymatic Cascade to the Plant Metabolitecis-(+)-12-OPDA and Metathesis-Derived Products. Adv. Sci. 2020, 7, 1902973. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Drouet, P.; Thomas, D.; Legoy, M.D. Production of 13(S)-hydroperoxy-9(Z),11(E)-octadecadienoic acid using soybean lipoxygenase 1 in a biphasic octane-water system. Tetrahedron Lett. 1994, 35, 3923–3926. [Google Scholar] [CrossRef]
- Márczy, J.S.; Németh, Á.S.; Samu, Z.; Háger-Veress, Á.; Szajáni, B. Production of hexanal from hydrolyzed sunflower oil by lipoxygenase and hydroperoxid lyase enzymes. Biotechnol. Lett. 2002, 24, 1673–1675. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Marlier, M. An efficient procedure for the production of fatty acid hydroperoxides from hydrolyzed flax seed oil and soybean lipoxygenase. Biotechnol. Tech. 1996, 10, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, A.S.; Marczy, J.S.; Samu, Z.; Hager-Veress, A.; Szajani, B. Biocatalytic production of 2(E)-hexenal from hydrolysed linseed oil. Enzym. Microb. Technol. 2004, 34, 667–672. [Google Scholar] [CrossRef]
- Piazza, G. Lipoxygenase catalyzed hydroperoxide formation in microemulsions containing nonionic surfactant. Biotechnol. Lett. 1992, 14, 1153–1158. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Maślakiewicz, P.; Haber, J. The effect of Linoleic Acid on pH Inside Sodium bis(2-ethylhexyl) sulfosuccinate Reverse Micelles in Isooctane and on the Enzymic Activity of Soybean Lipoxygenase. JBIC J. Biol. Inorg. Chem. 1996, 238, 549–553. [Google Scholar] [CrossRef]
- Kermasha, S.; Dioum, N.; Bisakowski, B. Biocatalysis of lipoxygenase in selected organic solvent media. J. Mol. Catal. B Enzym. 2001, 11, 909–919. [Google Scholar] [CrossRef]
- Gargouri, M.; Legoy, M.D. Bienzymatic reaction for hydroperoxide production in a multiphasic system. Enzym. Microb. Technol. 1997, 21, 79–84. [Google Scholar] [CrossRef]
- Gargouri, M.; Legoy, M.D. A two-enzyme system for the transformation of unsaturated oils to 9(S)-hydroperoxy fatty acids. Biotechnol. Lett. 2002, 24, 915–918. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; He, Y.; Liu, X.; Wang, P.; Xu, L.; Yan, J.; Yan, Y. Bi-enzyme directed self-assembled system toward biomimetic synthesis of fatty acid hydroperoxides like soybean. Compos. Part. B: Eng. 2021, 222, 109091. [Google Scholar] [CrossRef]
- Kim, K.-R.; An, J.-U.; Lee, S.-H.; Oh, D.-K. Selective Production of 9R-Hydroxy-10E,12Z,15Z-Octadecatrienoic Acid from α-Linolenic Acid in Perilla Seed Oil Hydrolyzate by a Lipoxygenase from Nostoc Sp. SAG 25.82. PLoS ONE 2015, 10, e0137785. [Google Scholar] [CrossRef] [Green Version]
- Elshof, M.; Janssen, M.; Veldink, G.; Vliegenthart, J. Biocatalytic large-scale production of 13(S)-hydroperoxy-9(Z), 11(E) octadecadienoic acid from hydrolysed safflower oil by a crude soybean-flour extract as lipoxygenase source. Recl. Trav. Chim. Pays-Bas 1996, 115, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Berry, H.; Debat, H.; Larreta-Garde, V. Excess substrate inhibition of soybean lipoxygenase-1 is mainly oxygen-dependent. FEBS Lett. 1997, 408, 324–326. [Google Scholar] [CrossRef] [Green Version]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Von Rybinski, W.; Hill, K. Alkyl polyglycosides—Properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Heo, S.-H.; Hou, C.T.; Kim, B.S. Production of oxygenated fatty acids from vegetable oils by Flavobacterium sp. strain DS5. New Biotechnol. 2009, 26, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Z.; Zheng, S.; Xu, H.; Zhou, Y.J.; Gao, Z.; Meng, C.; Li, S. Establishing an enzyme cascade for one-pot production of α-olefins from low-cost triglycerides and oils without exogenous H2O2 addition. Biotechnol. Biofuels 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.-Y.; Seo, J.-H.; Kang, W.-R.; Kim, M.-J.; Lee, J.-H.; Oh, D.-K.; Park, J.-B. Simultaneous Enzyme/Whole-Cell Biotransformation of Plant Oils into C9 Carboxylic Acids. ACS Catal. 2016, 6, 7547–7553. [Google Scholar] [CrossRef]
- Gargouri, M.; Legoy, M.D. Chemoenzymatic production of (+)-coriolic acid from trilinolein: Coupled synthesis and extraction. J. Am. Oil Chem. Soc. 1997, 74, 641–645. [Google Scholar] [CrossRef]
- Stolterfoht, H.; Rinnofner, C.; Winkler, M.; Pichler, H. Recombinant Lipoxygenases and Hydroperoxide Lyases for the Synthesis of Green Leaf Volatiles. J. Agric. Food Chem. 2019, 67, 13367–13392. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Dorscheid, S.; Witte, K.; Giffhorn, F.; Heinzle, E. Controlled feeding of hydrogen peroxide as oxygen source improves production of 5-ketofructose from L-sorbose using engineered pyranose 2-oxidase fromPeniophora gigantea. Biotechnol. Bioeng. 2012, 109, 2941–2945. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mannsberger, A.; Thomsen, M.S.; Tekautz, G.; Nidetzky, B. Process intensification for O2 -dependent enzymatic transformations in continuous single-phase pressurized flow. Biotechnol. Bioeng. 2018, 116, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.R.; Cosgrove, S.C.; Turner, N.J.; Kapur, N.; Blacker, A.J. Highly Productive Oxidative Biocatalysis in Continuous Flow by Enhancing the Aqueous Equilibrium Solubility of Oxygen. Angew. Chem. Int. Ed. 2018, 57, 10535–10539. [Google Scholar] [CrossRef] [Green Version]
- Beverungen, C.; Buyukuslu, N.; Candar, V.; Eryasa, Y.; Schörken, U.; Weiss, A.; Wunderlich, M. Process for recovering hydroperoxides. European Patent application EP 1336659A2, 7 February 2003. [Google Scholar]
- Pérez-Gilabert, M.; Veldink, G.A.; Vliegenthart, J.F.G. Protection by different agents against inactivation of lipoxygenase by hydrogen peroxide. Lipids 1996, 31, 1245–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollmann, T.; Zerhusen, C.; Glüsen, B.; Schörken, U. Structures and Properties of Sophorolipids in Dependence of Microbial Strain, Lipid Substrate and Post-Modification. Tenside Surfactants Deterg. 2019, 56, 367–377. [Google Scholar] [CrossRef]






| Surfactant Concentration: | 3% | 6% | ||
|---|---|---|---|---|
| Surfactant | HLB | Structure | Yield (%) | Yield (%) |
| Brij 93 | 4 | Polyethylenglykol-oleylether | 28.7 | 29.6 |
| Span 80 | 4.3 | Sorbitanmonooleat | 25.5 | 24.7 |
| Sophorolipid18:2 | ~6 | Linoleic acid glucoside | 26.2 | 28.4 |
| Tergitol 15-S-3 | 8.0 | Secondary Alcohol Ethoxylates | 28.5 | 29.7 |
| Ecosurf SA-9 | 11.1 | Seed oil alcohols ethoxylated propoxylated | 26.0 | 28.3 |
| Brij O10 | 12.4 | Polyoxyethylen-10-oleylether | 27.1 | 27.8 |
| Ecosurf EH-9 | 12.5 | Ethyl hexanol ethoxylated propoxylated | 33.3 | 29.9 |
| Tergitol NP 9 | 12.9 | Alkylphenol Ethoxylate (APE) | 26.2 | 30.3 |
| Triton CG-110 | ~13 | Alkyl Polyglucoside | 36.8 | 32.6 |
| Tergitol 15-S-9 | 13.3 | Secondary Alcohol Ethoxylates | 27.6 | 28.3 |
| Tergitol NP 10 | 13.3 | Alkylphenol Ethoxylate (APE) | 21.9 | 24.9 |
| Triton X-100 | 13.4 | Octyl phenol ethoxylate | 28.5 | 27.8 |
| Tween 80 | 15 | Polyoxyethylene (20) sorbitan monooleate | 23.9 | 23.7 |
| Tergitol 15-S-20 | 15.6 | Secondary Alcohol Ethoxylates | 31.5 | 20.9 |
| Tween 20 | 16.7 | Polyoxyethylene (20) sorbitan monolaurate | 16.5 | 30.8 |
| Tergitol 15-S-40 | 18.0 | Secondary Alcohol Ethoxylates | 23.0 | 26.5 |
| Substrate | Linoleic Acid | Linoleic Acid | Safflower Oil | Safflower Oil | Safflower Oil |
|---|---|---|---|---|---|
| Enzyme Addition | LOX-1 Direct | LOX-1 Dosed | LOX-1 Direct | LOX-1 Dosed | Lipase Only |
| (▲) | (●) | +Lipase (♦) | +Lipase (■) | (Control) | |
| Acid value | 176 ± 15 | 184 ± 9 | 23.5 ± 0.2 | 134 ± 9 | 160 ± 2 |
| HPODE/linoleic acid ratio | 67:33 | 64:36 | 61:39 | 84:16 | - |
| Degree of hydrolysis | - | - | 12 ± 0.1 | 72 ± 5 | 80 ± 1 |
| Parameter | Range/Compounds |
|---|---|
| Temperature | 20–30 °C |
| pH-value | 6.5–10 |
| Solvents | Methanol, Ethanol, DMSO, MTBE, Acetone, Acetonitrile, Isopropanol, 1-Butanol, Heptane, 1-Octanol |
| Detergents | Tween 20, Tween 80, Tergitol 15-s-3, Tergitol 15-s-7, Tergitol 15-s-9, Tergitol 15-s-20, Tergitol 15-s-40, Tergitol NP-9, Tergitol NP-10, Ecosurf SA-9, Ecosurf EH-9, Triton X-100, Triton CG-110, Sophorolipid C18:2 [30], Brij 93, Brij O 10, Span 80 |
| Oxygen supply | 0 to 100 mL/min |
| Substrate concentration | 1 to 100 mM (linoleic acid equivalent) |
| Substrate–enzyme ratio | LOX: 1000 to 1,000,000 U/mmol |
| Lipases | Amano AY 30 (C. rugosa) (172.8 ± 33.4 mg/g), Amano A (A. niger) (173.9 ± 15.4 mg/g), Amano F-AP 15 (30.8 ± 4.9 mg/g), Amano M (M. javanicus) (256.9 ± 11.5 mg/g), Amano (P. fluorescens) (109.4 ± 12 mg/g), Amano G (P. camemberti) (80.4 ± 5.3 mg/g), Amano PS (B. cepacia) (15.1 ± 3.1 mg/g), Lipase CCL (C. rugosa) (124.4 ± 17.3 mg/g), Palatase 20,000 L (45.3 ± 4.3 mg/g), Novozymes Lipolase 100 L (T. lanugenosis) (13.2 ± 1.4 mg/g), Novozymes Eversa Transform 2.0 (19.8 ± 1.9 mg/g), Novozymes CalB L (C. antarctica) (37.8 ± 0.6 mg/g) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gala Marti, V.; Coenen, A.; Schörken, U. Synthesis of Linoleic Acid 13-Hydroperoxides from Safflower Oil Utilizing Lipoxygenase in a Coupled Enzyme System with In-Situ Oxygen Generation. Catalysts 2021, 11, 1119. https://doi.org/10.3390/catal11091119
Gala Marti V, Coenen A, Schörken U. Synthesis of Linoleic Acid 13-Hydroperoxides from Safflower Oil Utilizing Lipoxygenase in a Coupled Enzyme System with In-Situ Oxygen Generation. Catalysts. 2021; 11(9):1119. https://doi.org/10.3390/catal11091119
Chicago/Turabian StyleGala Marti, Valentin, Anna Coenen, and Ulrich Schörken. 2021. "Synthesis of Linoleic Acid 13-Hydroperoxides from Safflower Oil Utilizing Lipoxygenase in a Coupled Enzyme System with In-Situ Oxygen Generation" Catalysts 11, no. 9: 1119. https://doi.org/10.3390/catal11091119