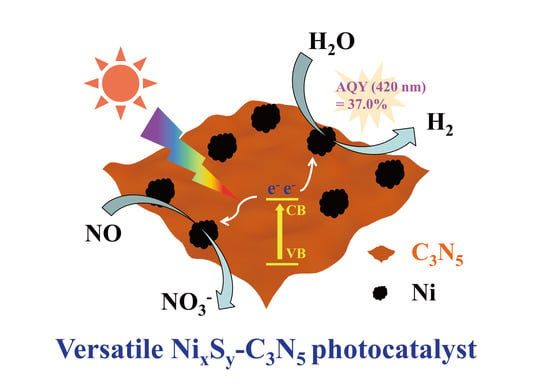
Noble-Metal-Free NixSy-C3N5 Hybrid Nanosheet with Highly Efficient Photocatalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Photocatalytic H2 Production Performance
2.3. Photocatalytic NO Oxidation Performance
3. Materials and Methods
3.1. Material Preparation
3.2. Catalyst Characterization
3.3. Photocatalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; He, C.; Fu, L.; Huo, J.; Zhao, C.; Li, X.; Song, Y. Capture and separation of CO2 on BC3 nanosheets: A DFT study. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Fu, L.; Wang, R.; Zhao, C.; Huo, J.; He, C.; Kim, K.; Zhang, W. Construction of Cr-embedded graphyne electrocatalyst for highly selective reduction of CO2 to CH4: A DFT study. Chem. Eng. J. 2021, 414, 128857. [Google Scholar] [CrossRef]
- Wang, R.; He, C.; Chen, W.; Zhao, C.; Huo, J. Rich B active centers in Penta-B2C as high-performance photocatalyst for nitrogen reduction. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Shen, R.; Ding, Y.; Li, S.; Zhang, P.; Xiang, Q.; Ng, Y.; Li, X. Constructing low-cost Ni3C/twin-crystal Zn0.5Cd0.5S heterojunction/homojunction nanohybrids for efficient photocatalytic H2 evolution. Chin. J. Catal. 2021, 42, 25–36. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi, A.; Jafer, R.; Li, X.; Zhang, P. A new heterojunction in photocatalysis: S-scheme heterojunction. Chin. J. Catal. 2021, 42, 667–669. [Google Scholar] [CrossRef]
- Wang, J.; Kuo, M.; Zeng, P.; Xu, L.; Chen, S.; Peng, T. Few-layer BiVO4 nanosheets decorated with SrTiO3: Rh nanoparticles for highly efficient visible-light-driven overall water splitting. Appl. Catal. B Environ. 2020, 279, 119377. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Q.; Zheng, Q.; Shen, R.; Zhang, P.; Li, X. Constructing 1D/2D Schottky-Based Heterojunctions between Mn0.2Cd0.8S Nanorods and Ti3C2 Nanosheets for Boosted Photocatalytic H2 Evolution. Acta Phys. Chim. Sin. 2021, 37, 2010059. [Google Scholar]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.; Zhang, P.; Li, X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Sun, W.; Xiang, Y.; Jiang, Z.; Wang, S.; Yang, N.; Jin, S.; Sun, L.; Teng, H.; Chen, H. Designed polymeric conjugation motivates tunable activation of molecular oxygen in heterogeneous organic photosynthesis. Sci. Bull. 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Chen, H.; Wang, S. Direct catalytic nitrogen oxide removal using thermal, electrical or solar energy. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; et al. In Situ Carbon Homogeneous Doping on Ultrathin Bismuth Molybdate: A Dual-Purpose Strategy for Efficient Molecular Oxygen Activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Antonietti, M.; Zhu, B.; Heil, T.; Yu, J.; Cao, S. Designing Defective Crystalline Carbon Nitride to Enable Selective CO2 Photoreduction in the Gas Phase. Adv. Funct. Mater. 2019, 29, 1900093. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.; Wang, B.; Chen, C.; Huang, Y.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.; Huang, Y.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Surface Engineering of g-C3N4 by Stacked BiOBr Sheets Rich in Oxygen Vacancies for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 4519–4524. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, B.; Tang, Z.; Ao, Z.; Xia, D.; Zhu, M.; Wang, S. Experimental and DFT insights into the visible-light driving metal-free C3N5 activated persulfate system for efficient water purification. Appl. Catal. B Environ. 2021, 289, 120023. [Google Scholar] [CrossRef]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.; Kar, P.; Alam, K.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.; Michaelis, V.; et al. C3N5: A Low Bandgap Semiconductor Containing an Azo-Linked Carbon Nitride Framework for Photocatalytic, Photovoltaic and Adsorbent Applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, Q.; Liu, M.; Yin, P.; Wu, C.; Li, H.; Zhang, Y.; Yao, S. Photoinduced Charge Separation via the Double-Electron Transfer Mechanism in Nitrogen Vacancies g-C3N5/BiOBr for the Photoelectrochemical Nitrogen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 38266–38274. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kim, S.; Jin, X.; Premkumar, S.; Chandra, G.; Lee, N.S.; Mane, G.P.; Hwang, S.J.; Umapathy, S.; Vinu, A. Ordered mesoporous C3N5 with a combined triazole and triazine framework and its graphene hybrids for the oxygen reduction reaction (ORR). Angew. Chem. Int. Ed. 2018, 57, 17381–17386. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Lakhi, K.S.; Ilbeygi, H.; Ravon, U.; Al-Bahily, K.; Mori, T.; Park, D.H.; Vinu, A. Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2017, 56, 8481–8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Yang, G.; Wang, W.; Wang, C.; Wang, M.; Sun, X.; Xu, P.; Zhang, J. Preparation of C3N5 nanosheets with enhanced performance in photocatalytic methylene blue (MB) degradation and H2-evolution from water splitting. Environ. Res. 2020, 188, 109741. [Google Scholar] [CrossRef]
- Peng, C.; Han, L.; Huang, J.; Wang, S.; Zhang, X.; Chen, H. Comprehensive investigation on robust photocatalytic hydrogen production over C3N5. Chin. J. Catal. 2021. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zhang, X.; Hu, S.; Zhang, Z.; Zhou, W.; Liu, H. Multi-interface collaboration of graphene cross-linked NiS-NiS2-Ni3S4 polymorph foam towards robust hydrogen evolution in alkaline electrolyte. Nano Res. 2021. [Google Scholar] [CrossRef]
- Vijaya, S.; Landi, G.; Wu, J.J.; Anandan, S. Ni3S4/CoS2 mixed-phase nanocomposite as counter electrode for Pt-free dye-sensitized solar cells. J. Power Sources 2020, 478, 229068. [Google Scholar] [CrossRef]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. NixSy nanowalls/nitrogen-doped graphene foam is an efficient trifunctional catalyst for unassisted artificial photosynthesis. Adv. Funct. Mater. 2018, 28, 1706917. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy) hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, Y.S.; Lu, W.T.; He, D.; Wang, C.Y.; Li, Y.K.; Wang, X.Y.; Cao, F.F. Enhanced catalysis of electrochemical overall water splitting in alkaline media by Fe doping in Ni3S2 nanosheet arrays. ACS Catal. 2018, 8, 5431–5441. [Google Scholar] [CrossRef]
- Yan, A.; Shi, X.; Huang, F.; Fujitsuka, M.; Majima, T. Efficient photocatalytic H2 evolution using NiS/ZnIn2S4 heterostructures with enhanced charge separation and interfacial charge transfer. Appl. Catal. B Environ. 2019, 250, 163–170. [Google Scholar] [CrossRef]
- Wang, B.; Ding, Y.; Deng, Z.; Li, Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019, 40, 335–342. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Liu, J.; Shi, W.; Yang, G.; Wang, G.C.; Cheng, P. An Efficient, Visible-Light-Driven, Hydrogen Evolution Catalyst NiS/ZnxCd1− xS Nanocrystal Derived from a Metal–Organic Framework. Angew. Chem. Int. Ed. 2018, 130, 9938–9942. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Li, H.; Ma, S.; He, K.; Xu, Y.; Fang, Y.; Liu, W.; Gao, Q. Enhanced visible-light H2 evolution of g-C3N4 photocatalysts via the synergetic effect of amorphous NiS and cheap metal-free carbon black nanoparticles as co-catalysts. Appl. Surf. Sci. 2015, 358, 204–212. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Cui, G.; Dong, Y.; Wang, G.; Jiang, P.; Wu, X.; Zhao, N. A photochemical synthesis route to typical transition metal sulfides as highly efficient cocatalyst for hydrogen evolution: From the case of NiS/g-C3N4. Appl. Catal. B Environ. 2018, 225, 284–290. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Yang, N.; Zhan, G.; Zhang, X.; Dong, G.; Zhang, L.; Chen, H. Insight into the effect of bromine on facet-dependent surface oxygen vacancies construction and stabilization of Bi2MoO6 for efficient photocatalytic NO removal. Appl. Catal. B Environ. 2020, 265, 118585. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, A.; Cao, Y.; Wang, S.; Dong, F.; Zhou, Y. Mo-doped carbon nitride homojunction to promote oxygen activation for enhanced photocatalytic performance. Chem. Eng. J. 2020, 401, 126028. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Zheng, Q.; Cui, W.; Liu, Y.; Zheng, K.; Dong, F.; Zhou, Y. Dual functions of O-atoms in the g-C3N4/BO0.2N0.8 interface: Oriented charge flow in-plane and separation within the interface to collectively promote photocatalytic molecular oxygen activation. ACS Appl. Mater. Interfaces 2020, 12, 34432–34440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Chen, M.; Shi, X.; Zhang, Y.; Cao, J.; Ho, W.; Lee, S.C. Roles of N-vacancies over porous g-C3N4 microtubes during photocatalytic NOx removal. ACS Appl. Mater. Interfaces 2019, 11, 10651–10662. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Chen, T.; Yang, N.; Jiang, K.; Wang, P.; Li, S.; Ding, X.; Chen, H. Chloridion-induced dual tunable fabrication of oxygen-deficient Bi2WO6 atomic layers for deep oxidation of NO. Chen, Chin. J. Catal. 2021, 42, 1013–1023. [Google Scholar] [CrossRef]
- Kou, M.; Deng, Y.; Zhang, R.; Wang, L.; Wong, P.K.; Su, F.; Ye, L. Molecular oxygen activation enhancement by BiOBr0.5I0.5/BiOI utilizing the synergistic effect of solid solution and heterojunctions for photocatalytic NO removal. Chin. J. Catal. 2020, 41, 1480–1487. [Google Scholar] [CrossRef]
- Shi, X.; Wang, P.; Li, W.; Bai, Y.; Xie, H.; Zhou, Y.; Ye, L. Change in photocatalytic NO removal mechanisms of ultrathin BiOBr/BiOI via NO3–adsorption. Appl. Catal. B Environ. 2019, 243, 322–329. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Hou, M.; Xiang, Y.; Chen, H. Robust visible/near-infrared light driven hydrogen generation over Z-scheme conjugated polymer/CdS hybrid. Appl. Catal. B Environ. 2018, 224, 871–876. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Yu, L.; Li, R.; Li, Q.; Li, Z. Visible/near-infrared-light-induced H2 production over g-C3N4 co-sensitized by organic dye and zinc phthalocyanine derivative. ACS Catal. 2015, 5, 504–510. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]










| Photocatalysts | Reaction Conditions | H2 Production Rate μmol g−1 h−1 | Ref. |
|---|---|---|---|
| Pt/C3N5 | 300 W Xe lamp (λ > 420 nm), TEOA | 28,956 | [27] |
| NiS/ZnIn2S4 | 320 W Xe lamp (λ > 420 nm), Lactic Acid | 3333 | [33] |
| NiS/CQDs/ZnIn2S4 | 300 W Xe lamp (λ > 420 nm), TEOA | 568 | [34] |
| g-C3N4/1.5% NiS | 300 W Xe lamp (λ > 420 nm), TEOA | 395 | [36] |
| NiS/g-C3N4 | 300 W Xe lamp (λ > 420 nm), TEOA | 16,400 | [37] |
| NixSy-C3N5 | 300 W Xe lamp (λ > 420 nm), TEOA | 35,444 | this work |
| Photocatalysts | Reaction Conditions | NO Removal Efficiency (%) | Ref. |
|---|---|---|---|
| Mo-g-C3N4/g-C3N4 | flow reactor, metal halide lamp, >420 nm | 36.0 | [39] |
| g-C3N4/BO0.2N0.8 | flow reactor, metal halide lamp, >420 nm | 30.2 | [40] |
| N-Vacancies g-C3N4 | flow reactor, Xe lamp, >420 nm | 32.8 | [41] |
| NixSy-C3N5 | flow reactor, visible LED | 40.0 | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Peng, C.; Huang, J.; Sun, L.; Wang, S.; Zhang, X.; Yang, Y. Noble-Metal-Free NixSy-C3N5 Hybrid Nanosheet with Highly Efficient Photocatalytic Performance. Catalysts 2021, 11, 1089. https://doi.org/10.3390/catal11091089
Han L, Peng C, Huang J, Sun L, Wang S, Zhang X, Yang Y. Noble-Metal-Free NixSy-C3N5 Hybrid Nanosheet with Highly Efficient Photocatalytic Performance. Catalysts. 2021; 11(9):1089. https://doi.org/10.3390/catal11091089
Chicago/Turabian StyleHan, Lixiao, Cong Peng, Jinming Huang, Linhao Sun, Shengyao Wang, Xiaohu Zhang, and Yi Yang. 2021. "Noble-Metal-Free NixSy-C3N5 Hybrid Nanosheet with Highly Efficient Photocatalytic Performance" Catalysts 11, no. 9: 1089. https://doi.org/10.3390/catal11091089