Photoelectrochemical Degradation of Organic Pollutants on a La3+ Doped BiFeO3 Perovskite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphology Characterisation of the Electrodes
2.2. UV-VIS Diffuse Reflectance Spectroscopic Characterisation of the Photoanodes
2.3. Electrochemical and Photoelectrochemical Characterisation
2.4. Photoelectrocatalytic Degradation of Pollutants
2.5. Photoelectrocatalytic Degradation of Cocktail Pollutants and Pharmaceuticals
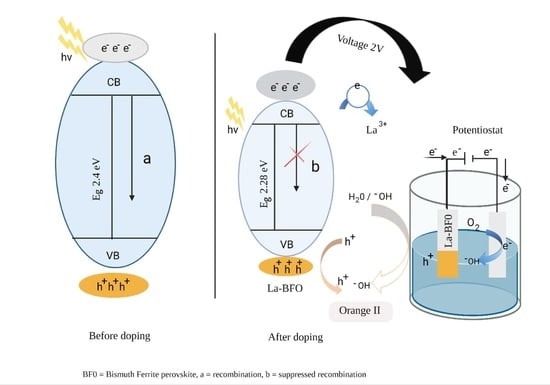
2.6. Trapping Experiment and Proposed Photoelectrocatalytic Activity Mechanism
3. Materials and Method
3.1. Preparation of Bi1−xLaxFeO3 Perovskite
3.2. Structural, Morphology and Optical Characterisation of the Prepared Electrodes
3.3. Preparation of Bi1−xLaxFeO3 Photoanodes for Electrochemical and Photoelectrochemical Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peleyeju, M.G.; Arotiba, O.A. Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1389–1411. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakrabarty, S.; Kumari, N.; Su, W.N.; Basu, S. Visible-Light-Mediated Electrocatalytic Activity in Reduced Graphene Oxide-Supported Bismuth Ferrite. ACS Omega 2018, 3, 5946–5957. [Google Scholar] [CrossRef]
- Kong, J.; Yang, T.; Rui, Z.; Ji, H. Perovskite-Based Photocatalysts for Organic Contaminants Removal: Current Status and Future Perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Venkatesh, G.; Geerthana, M.; Prabhu, S.; Ramesh, R.; Prabu, K.M. Enhanced Photocatalytic Activity of Reduced Graphene Oxide/SrSnO3 Nanocomposite for Aqueous Organic Pollutant Degradation. Optik 2020, 206, 164055. [Google Scholar] [CrossRef]
- Wang, F.; Chen, D.; Zhang, N.; Wang, S.; Qin, L.; Sun, X.; Huang, Y. Oxygen Vacancies Induced by Zirconium Doping in Bismuth Ferrite Nanoparticles for Enhanced Photocatalytic Performance. J. Colloid Interface Sci. 2017, 508, 237–247. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, X.; Marcel Veder, J.P.; Shao, Z. Recent Advances in Anion-Doped Metal Oxides for Catalytic Applications. J. Mater. Chem. A 2019, 7, 7280–7300. [Google Scholar] [CrossRef]
- Shawky, A.; Mohamed, R.M.; Mkhalid, I.A.; Youssef, M.A.; Awwad, N.S. Visible Light-Responsive Ag/LaTiO3 Nanowire Photocatalysts for Efficient Elimination of Atrazine Herbicide in Water. J. Mol. Liq. 2020, 299, 112163. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B. BiFeO3/BiVO4 p−n Heterojunction for e Ffi Cient and Stable Photocatalytic and Photoelectrochemical Water Splitting under Visible-Light Irradiation. Catal. Today 2020, 340, 188–196. [Google Scholar] [CrossRef]
- Ali, S.; Humayun, M.; Pi, W.; Yuan, Y.; Wang, M. Fabrication of BiFeO3-g-C3N4-WO3 Z-Scheme Heterojunction as Highly Efficient Visible-Light Photocatalyst for Water Reduction and 2,4-Dichlorphenol Degradation: Insight Mechanism. J. Hazard. Mater. 2020, 397, 122708. [Google Scholar] [CrossRef]
- Acharya, S.; Martha, S.; Sahoo, P.C.; Parida, K. Glimpses of the Modification of Perovskite with Graphene-Analogous Materials in Photocatalytic Applications. Inorganic Chemistry Frontiers. Inorg. Chem. Front. 2015, 2, 807–823. [Google Scholar] [CrossRef]
- Gade, R.; Ahemed, J.; Yanapu, K.L.; Abate, S.Y.; Tao, Y.T.; Pola, S. Photodegradation of Organic Dyes and Industrial Wastewater in the Presence of Layer-Type Perovskite Materials under Visible Light Irradiation. J. Environ. Chem. Eng. 2018, 6, 4504–4513. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Niu, F.; Zhang, N.; Qin, L.; Huang, Y. Pd Cocatalyst on Sm-Doped BiFeO3 Nanoparticles: Synergetic Effect of a Pd Cocatalyst and Samarium Doping on Photocatalysis. RSC Adv. 2016, 6, 34574–34587. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pal, M. Highly Efficient Novel Carbon Monoxide Gas Sensor Based on Bismuth Ferrite Nanoparticles for Environmental Monitoring. New J. Chem. 2018, 42, 7188–7196. [Google Scholar] [CrossRef]
- Pei, Y.L.; Zhang, C. Effect of Ion Doping in Different Sites on the Morphology and Photocatalytic Activity of BiFeO3 Microcrystals. J. Alloys Compd. 2013, 570, 57–60. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Zhu, Y.; Niu, F.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Synthesis of BiFeo3 Nanoparticles for the Visible-Light Induced Photocatalytic Property. Mater. Res. Bull. 2014, 59, 6–12. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Lei, M.; She, Y.; Cao, M.; Tang, H. Ligand-Induced Drastic Enhancement of Catalytic Activity of Nano-BiFeO3 for Oxidative Degradation of Bisphenol A. ACS Catal. 2011, 1, 1193–1202. [Google Scholar] [CrossRef]
- Dixit, T.K.; Sharma, S.; Sinha, A.S.K. Development of Heterojunction in N-RGO Supported Bismuth Ferrite Photocatalyst for Degradation of Rhodamine B. Inorg. Chem. Commun. 2020, 117, 107945. [Google Scholar] [CrossRef]
- Niu, F.; Chen, D.; Qin, L.; Zhang, N.; Wang, J.; Chen, Z.; Huang, Y. Facile Synthesis of Highly Efficient p-n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ChemCatChem 2015, 7, 3279–3289. [Google Scholar] [CrossRef]
- Niu, F.; Chen, D.; Qin, L.; Gao, T.; Zhang, N.; Wang, S.; Chen, Z.; Wang, J.; Sun, X.; Huang, Y. Synthesis of Pt/BiFeO3 Heterostructured Photocatalysts for Highly Efficient Visible-Light Photocatalytic Performances. Sol. Energy Mater. Sol. Cells 2015, 143, 386–396. [Google Scholar] [CrossRef]
- Kumar, P.; Chand, P.; Singh, V. La3+ Substituted BiFeO3-a Proficient Nano Ferrite Photo-Catalyst under the Application of Visible Light. Chem. Phys. Lett. 2020, 754, 137715. [Google Scholar] [CrossRef]
- Kazhugasalamoorthy, S.; Jegatheesan, P.; Mohandoss, R.; Giridharan, N.V.; Karthikeyan, B.; Joseyphus, R.J.; Dhanuskodi, S. Investigations on the Properties of Pure and Rare Earth Modified Bismuth Ferrite Ceramics. J. Alloys Compd. 2010, 493, 569–572. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Yu, Y.; Liu, J.; Pei, L.; Wang, J.; Liu, X.; Yu, B.; Zhao, X. Effects of Nd and High-Valence Mn Co-Doping on the Electrical and Magnetic Properties of Multiferroic BiFeO3 Ceramics. Solid State Commun. 2010, 150, 1088–1091. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Li, A.H.; Wang, X.L.; Dou, S.X.; Ozawa, K.; Kimura, H.; Zhang, S.J.; Shrout, T.R. Structure, Ferroelectric Properties, and Magnetic Properties of the La-Doped Bismuth Ferrite. J. Appl. Phys. 2008, 103, 137–140. [Google Scholar] [CrossRef]
- Meng, W.; Hu, R.; Yang, J.; Du, Y.; Li, J.; Wang, H. Influence of Lanthanum-Doping on Photocatalytic Properties of BiFeO3 for Phenol Degradation. Chin. J. Catal. 2016, 37, 1283–1292. [Google Scholar] [CrossRef]
- Dhanalakshmi, R.; Muneeswaran, M.; Shalini, K.; Giridharan, N.V. Enhanced Photocatalytic Activity of La-Substituted BiFeO3 Nanostructures on the Degradation of Phenol Red. Mater. Lett. 2016, 165, 205–209. [Google Scholar] [CrossRef]
- Sharma, S.; Saravanan, P.; Pandey, O.P.; Vinod, V.T.P.; Černík, M.; Sharma, P. Magnetic Behaviour of Sol-Gel Driven BiFeO3 Thin Films with Different Grain Size Distribution. J. Magn. Magn. Mater. 2016, 401, 180–187. [Google Scholar] [CrossRef]
- Delfard, N.B.; Maleki, H. Enhanced Structural, Optical, and Multiferroic Properties of Rod-Like Bismuth Iron Oxide Nanoceramics by Dopant Lanthanum. J. Supercond. Nov. Magn. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Gholam, T.; Ablat, A.; Mamat, M.; Wu, R.; Aimidula, A.; Bake, M.A.; Zheng, L.; Wang, J.; Qian, H.; Wu, R.; et al. Local Electronic Structure Analysis of Zn-Doped BiFeO3 Powders by X-Ray Absorption Fine Structure Spec-troscopy. J. Alloys Compd. 2017, 710, 843–849. [Google Scholar] [CrossRef]
- Jiang, Y.; Ning, H.; Yu, J. Optical Bandgap Tuning of Ferroelectric Semiconducting BiFeO3-Based Oxide Perovskites via Chemical Substitution for Photovoltaics. AIP Adv. 2018, 8, 125334. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hu, R.; Meng, W.; Du, Y. A Novel P-LaFeO3/n-Ag3PO4 Heterojunction Photocatalyst for Phenol Degradation under Visible Light Irradiation. Chem. Commun. 2016, 52, 2620–2623. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; He, J.; He, Y.; Wang, D. One-Step Hydrothermal Method to Prepare Nitrogen and Lanthanum Co-Doped TiO2 Nanocrystals with Exposed {0 0 1} Facets and Study on Their Photocatalytic Activities in Visible Light. J. Alloys Compd. 2015, 637, 308–314. [Google Scholar] [CrossRef]
- Malathi, A.; Arunachalam, P.; Kirankumar, V.S.; Madhavan, J.; Al-Mayouf, A.M. An Efficient Visible Light Driven Bismuth Ferrite Incorporated Bismuth Oxyiodide (BiFeO3/BiOI) Composite Photocatalytic Material for Degradation of Pollutants. Opt. Mater. 2018, 84, 227–235. [Google Scholar] [CrossRef]
- Khoomortezaei, S.; Abdizadeh, H.; Golobostanfard, M.R. Triple Layer Heterojunction WO3/BiVO4/BiFeO3 Porous Photoanode for E Ffi Cient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2019, 2, 6428–6439. [Google Scholar] [CrossRef]
- Koiki, B.A.; Orimolade, B.O.; Zwane, B.N.; Nkwachukwu, O.V.; Muzenda, C.; Nkosi, D.; Arotiba, O.A. The Application of FTO-Cu2O/Ag3PO4 Heterojunction in the Photoelectrochemical Degradation of Emerging Pharmaceutical Pollutant under Visible Light Irradiation. Chemosphere 2021, 266, 129231. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Soltani, T.; Lee, B. Modified Bismuth Vanadate by Bismuth Ferrite. New J. Chem. 2019, 43, 9106–9115. [Google Scholar] [CrossRef]
- Hussain, T.; Siddiqi, S.A.; Atiq, S.; Awan, M.S. Induced Modifications in the Properties of Sr Doped BiFeO3 Multiferroics. Prog. Nat. Sci. Mater. Int. 2013, 23, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Orimolade, B.O.; Koiki, B.A.; Peleyeju, G.M.; Arotiba, O.A. Visible Light Driven Photoelectrocatalysis on a FTO/BiVO4/BiOI Anode for Water Treatment Involving Emerging Pharmaceutical Pollutants. Electrochim. Acta 2019, 307, 285–292. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Zwane, B.N.; Koiki, B.A.; Tshwenya, L.; Peleyeju, G.M.; Mabuba, N.; Zhou, M.; Arotiba, O.A. Solar Photoelectrocatalytic Degradation of Ciprofloxacin at a FTO/BiVO4/MnO2 Anode: Kinetics, Intermediate Products and Degradation Pathway Studies. J. Environ. Chem. Eng. 2020, 8, 103607. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Fulgione, I.; Frontistis, Z.; Rizzo, L.; Mantzavinos, D. Solar Light-Induced Photoelectrocatalytic Degradation of Bisphenol-A on TiO2/ITO Film Anode and BDD Cathode. Catal. Today 2013, 209, 74–78. [Google Scholar] [CrossRef]
- Frontistis, Z.; Daskalaki, V.M.; Katsaounis, A.; Poulios, I.; Mantzavinos, D. Electrochemical Enhancement of Solar Photocatalysis: Degradation of Endocrine Disruptor Bisphenol-A on Ti/TiO2 Films. Water Res. 2011, 45, 2996–3004. [Google Scholar] [CrossRef]
- Khosla, E.; Kaur, S.; Dave, P.N. Mechanistic Study of Adsorption of Acid Orange-7 over Aluminum Oxide Nanoparticles. J. Eng. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. Rapid Decolorization of Acid Orange II Aqueous Solution by Amorphous Zero-Valent Iron. J. Environ. Sci. 2012, 24, 1021–1026. [Google Scholar] [CrossRef]
- Han, S.; Li, J.; Yang, K.; Lin, J. Fabrication of a β-Bi2O3/BiOI Heterojunction and Its Efficient Photocatalysis for Organic Dye Removal. Chin. J. Catal. 2015, 36, 2119–2126. [Google Scholar] [CrossRef]
- Wang, T.; Lang, J.; Zhao, Y.; Su, Y.; Zhao, Y.; Wang, X. Simultaneous Doping and Heterojunction of Silver on Na2Ta2O6 Nanoparticles for Visible Light Driven Photocatalysis: The Relationship between Tunable Optical Absorption, Defect Chemistry and Photocatalytic Activity. CrystEngComm 2015, 17, 6651–6660. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of Novel Magnetically Separable Nanocomposites Using Graphitic Carbon Nitride, Silver Phosphate and Silver Chloride and Their Applications in Photocatalytic Removal of Different Pollutants Using Visible-Light Irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.S.R.K. Tungsten-Based Nanomaterials (WO3 & Bi2WO6): Modifications Related to Charge Carrier Transfer Mechanisms and Photocatalytic Applications. Appl. Surf. Sci. 2015, 355, 939–958. [Google Scholar]
- Kostyukhin, E.M.; Kustov, A.L.; Kustov, L.M. One-Step Hydrothermal Microwave-Assisted Synthesis of LaFeO3 Nanoparticles. Ceram. Int. 2019, 45, 14384–14388. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkwachukwu, O.V.; Muzenda, C.; Ojo, B.O.; Zwane, B.N.; Koiki, B.A.; Orimolade, B.O.; Nkosi, D.; Mabuba, N.; Arotiba, O.A. Photoelectrochemical Degradation of Organic Pollutants on a La3+ Doped BiFeO3 Perovskite. Catalysts 2021, 11, 1069. https://doi.org/10.3390/catal11091069
Nkwachukwu OV, Muzenda C, Ojo BO, Zwane BN, Koiki BA, Orimolade BO, Nkosi D, Mabuba N, Arotiba OA. Photoelectrochemical Degradation of Organic Pollutants on a La3+ Doped BiFeO3 Perovskite. Catalysts. 2021; 11(9):1069. https://doi.org/10.3390/catal11091069
Chicago/Turabian StyleNkwachukwu, Oluchi V., Charles Muzenda, Babatope O. Ojo, Busisiwe N. Zwane, Babatunde A. Koiki, Benjamin O. Orimolade, Duduzile Nkosi, Nonhlangabezo Mabuba, and Omotayo A. Arotiba. 2021. "Photoelectrochemical Degradation of Organic Pollutants on a La3+ Doped BiFeO3 Perovskite" Catalysts 11, no. 9: 1069. https://doi.org/10.3390/catal11091069