A Comprehensive Assessment of Catalytic Performances of Mn2O3 Nanoparticles for Peroxymonosulfate Activation during Bisphenol A Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Particle Sizes on Degradation of BPA in Mn2O3/PMS System
2.2. Effect of PMS and Catalyst Dosages
2.3. Influence of Water Matrix on the Degradation of BPA
2.3.1. Effect of pH
2.3.2. Effect of Co-existing Inorganic Ions
2.3.3. Effects of HA and BPA Initial Concentrations
2.3.4. Degradation of BPA in Real Water and in the Presence of other EOCs
2.4. Detection of Reactive Species and Possible Generation Mechanisms
2.4.1. Identification of Reactive Species
2.4.2. Roles of Manganese and Oxygen Species
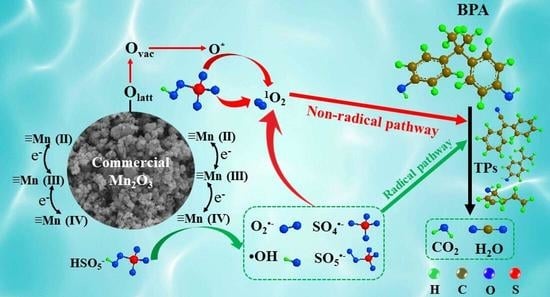
2.4.3. Hypothetic Mechanisms of Reactive Oxygen Species Generation
2.5. Degradation Pathways of BPA in the Mn2O3/PMS System
2.6. Comparison between Mn2O3 and other Lab-Made Mn-Based Catalysts
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Catalytic Degradation Experiments
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chem. Eng. J. 2018, 338, 300–310. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhou, Q.; Huang, X. Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater. 2019, 384, 121488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Li, J.; Yang, M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2018, 655, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-X.; Wang, C.-M.; Cao, J.-k.; Cao, W.-X.; Xu, Q.; Li, J. Monitoring three typical phenol endocrine disrupting compounds in drinking water of Suzhou urban area—From raw water to tap water. Int. J. Environ. Anal. Chem. 2018, 98, 921–937. [Google Scholar] [CrossRef]
- Tang, S.; He, C.; Thai, P.K.; Heffernan, A.; Vijayasarathy, S.; Toms, L.; Thompson, K.; Hobson, P.; Tscharke, B.J.; O’Brien, J.W.; et al. Urinary Concentrations of Bisphenols in the Australian Population and Their Association with the Per Capita Mass Loads in Wastewater. Environ. Sci. Technol. 2020, 54, 10141–10148. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Genc, B. Bisphenol A treatment by the hot persulfate process: Oxidation products and acute toxicity. J. Hazard. Mater. 2013, 263, 283–290. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.C.; Lam, P.K.; Moon, H.-B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, W.; Tan, Y.; Zhang, X. Emerging organic contaminants and odorous compounds in secondary effluent wastewater: Identification and advanced treatment. J. Hazard. Mater. 2020, 408, 124817. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global assessment of bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Yang, S.; Dzakpasu, M.; Li, X.; Ding, D.; Jin, P.; Chen, R.; Zhang, Q.; Wang, X. Attenuation of BPA degradation by SO4− in a system of peroxymonosulfate coupled with Mn/Fe MOF-templated catalysts and its synergism with Cl− and bicarbonate. Chem. Eng. J. 2019, 372, 605–615. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2019, 384, 121350. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2018, 356, 904–914. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Liu, J.; Chen, M.; Ahmed, Z.; Zhu, N. Efficient removal of bisphenol A by superoxide radical and singlet oxygen generated from peroxymonosulfate activated with Fe0-montmorillonite. Chem. Eng. J. 2018, 350, 484–495. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Sun, Z.; Li, C.; Zhang, X.; Kong, M.; Zheng, S.; Dionysiou, D.D. Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for bisphenol A degradation. Appl. Catal. B Environ. 2019, 253, 206–217. [Google Scholar] [CrossRef]
- Xue, S.; Li, Q.; Wang, L.; You, W.; Zhang, J.; Che, R. Copper- and Cobalt-Codoped CeO2 Nanospheres with Abundant Oxygen Vacancies as Highly Efficient Electrocatalysts for Dual-Mode Electrochemical Sensing of MicroRNA. Anal. Chem. 2019, 91, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Kang, S.; Xie, X.; Liao, C.; Duan, X.; Dionysiou, D.D. Efficient degradation of bisphenol A in water by heterogeneous activation of peroxymonosulfate using highly active cobalt ferrite nanoparticles. J. Hazard. Mater. 2020, 399, 122979. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.; Qiu, C.; Chen, Y.; Ma, X.; Gao, N.; Li, X. Magnetic MnFe2O4 activated peroxymonosulfate processes for degradation of bisphenol A: Performance, mechanism and application feasibility. Appl. Surf. Sci. 2018, 459, 138–147. [Google Scholar] [CrossRef]
- Li, W.; Wu, P.-X.; Zhu, Y.; Huang, Z.; Lu, Y.-H.; Li, Y.-W.; Dang, Z.; Zhu, N.-W. Catalytic degradation of bisphenol A by CoMnAl mixed metal oxides catalyzed peroxymonosulfate: Performance and mechanism. Chem. Eng. J. 2015, 279, 93–102. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.; Zhang, Q.; Zhang, L.; Yang, L.; Ding, D.; Wang, W.; et al. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Zeng, T.; Wang, H.; Sun, Y.; Chen, L.; Song, S.; Shi, H. Stable incorporation of MnOx quantum dots into N-doped hollow carbon: A synergistic peroxymonosulfate activator for enhanced removal of bisphenol A. Sep. Purif. Technol. 2018, 213, 264–275. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, T.; Wang, N.; Ma, W.; Sun, B.; Chu, J.; Lin, K.-Y.A.; Du, Y. Facile Synthesis of Yolk–Shell Mn3O4 Microspheres as a High-Performance Peroxymonosulfate Activator for Bisphenol A Degradation. Ind. Eng. Chem. Res. 2019, 58, 21304–21311. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Kim, S.H.; Dionysiou, D.D. Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation. Chem. Eng. J. 2019, 376, 119193. [Google Scholar] [CrossRef]
- Luo, X.; Liang, H.; Qu, F.; Ding, A.; Cheng, X.; Tang, C.Y.; Li, G. Free-standing hierarchical α-MnO2@ CuO membrane for catalytic filtration degradation of organic pollutants. Chemosphere 2018, 200, 237–247. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, Y.; Lu, F.; Wei, F.; Wang, X.; Wang, S. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef]
- Tian, N.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Li, Y. Enhanced 2, 4-dichlorophenol degradation at pH 3–11 by peroxymonosulfate via controlling the reactive oxygen species over Ce substituted 3D Mn2O3. Chem. Eng. J. 2018, 355, 448–456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Niu, C.; Tang, N.; Guo, H.; Wen, X.-J.; Liang, C.; Zeng, G. Enhanced activation of peroxymonosulfate by magnetic Co3MnFeO6 nanoparticles for removal of carbamazepine: Efficiency, synergetic mechanism and stability. Chem. Eng. J. 2019, 362, 851–864. [Google Scholar] [CrossRef]
- Huang, Y.; Nengzi, L.-C.; Zhang, X.; Gou, J.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism. Chem. Eng. J. 2020, 388, 124274. [Google Scholar] [CrossRef]
- Deng, J.; Ya, C.; Ge, Y.; Cheng, Y.; Chen, Y.; Xu, M.; Wang, H. Activation of peroxymonosulfate by metal (Fe, Mn, Cu and Ni) doping ordered mesoporous Co3O4 for the degradation of enrofloxacin. RSC Adv. 2018, 8, 2338–2349. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.-X.; Wang, C.-Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants. Sep. Purif. Technol. 2018, 213, 456–464. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Liang, S.; Shan, L.; Xie, X.; Yi, Z.; Wang, Y.; Guo, S.; Zhou, J. Oxygen Defects in β-MnO2 Enabling High-Performance Rechargeable Aqueous Zinc/Manganese Dioxide Battery. Iscience 2020, 23, 100797. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Xue, Q.; Yu, K.; Li, R.; Jiang, D.; Ge, L.; Ren, Y.; Chen, C.; Wu, X. Superior strength-ductility synergy by hetero-structuring high manganese steel. Mater. Res. Lett. 2020, 8, 417–423. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Ge, Y.; Tan, C.; Wang, H.; Li, Q.; Zhou, S.; Zhang, K. Degradation of ciprofloxacin using α-MnO2 activated peroxymonosulfate process: Effect of water constituents, degradation intermediates and toxicity evaluation. Chem. Eng. J. 2017, 330, 1390–1400. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Pang, S.-Y.; Zhou, Y.; Li, J.; Sun, S.; Gao, Y.; Jiang, C. Oxidation of bisphenol A by nonradical activation of peroxymonosulfate in the presence of amorphous manganese dioxide. Chem. Eng. J. 2018, 352, 1004–1013. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M= Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B Environ. 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Deng, Y.; Zhang, T.; Shih, K. Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms. Environ. Sci. Technol. 2016, 50, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.-J. Heterogeneous activation of peroxymonosulfate by a novel magnetic 3D γ-MnO2@ ZnFe2O4/rGO nanohybrid as a robust catalyst for phenol degradation. Appl. Catal. B Environ. 2019, 244, 946–956. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, Y.-F.; Huang, C.-i.; Chen, C.-Y. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J. Hazard. Mater. 2009, 170, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ghanbari, F. Combination of UVC-LEDs and ultrasound for peroxymonosulfate activation to degrade synthetic dye: Influence of promotional and inhibitory agents and application for real wastewater. Environ. Sci. Pollut. Res. 2017, 25, 6003–6014. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Hu, Z.-T.; Lim, T.-T. A novel quasi-cubic CuFe2O4–Fe2O3 catalyst prepared at low temperature for enhanced oxidation of bisphenol A via peroxymonosulfate activation. J. Mater. Chem. A 2015, 3, 22208–22217. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Lou, X.; Fang, C.; Geng, Z.; Jin, Y.; Xiao, D.; Wang, Z.; Liu, J.; Guo, Y. Significantly enhanced base activation of peroxymonosulfate by polyphosphates: Kinetics and mechanism. Chemosphere 2017, 173, 529–534. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Hong, J.; Dong, Z.; Wang, J. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4–x. Chemosphere 2019, 221, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhang, W.; Yan, C.; Xu, W.; Wu, L.; Ye, Y.; Hu, Y.; Dong, W. Enhanced removal of organic contaminants in water by the combination of peroxymonosulfate and carbonate. Sci. Total. Environ. 2018, 647, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Mamba, B.; Krause, R.; Malefetse, T.; Sithole, S.; Nkambule, T.T. Humic acid as a model for natural organic matter (NOM) in the removal of odorants from water by cyclodextrin polyurethanes. Water SA 2012, 35, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B Environ. 2019, 256, 117783. [Google Scholar] [CrossRef]
- Nie, M.; Deng, Y.; Nie, S.; Yan, C.; Ding, M.; Dong, W.; Dai, Y.; Zhang, Y. Simultaneous removal of bisphenol A and phosphate from water by peroxymonosulfate combined with calcium hydroxide. Chem. Eng. J. 2019, 369, 35–45. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.-H.; Zhang, J.; Huang, R.-P.; Yin, H.; Dang, Z.; Wu, P.-X.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2018, 53, 307–315. [Google Scholar] [CrossRef]
- Jawad, A.; Lu, X.; Chen, Z.; Yin, G. Degradation of Chlorophenols by Supported Co–Mg–Al Layered Double Hydrotalcite with Bicarbonate Activated Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 10028–10035. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Kong, L.; Lu, W.; Natarajan, V.; Zhu, F.; Zhan, J. Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation. Chem. Eng. J. 2018, 360, 1213–1222. [Google Scholar] [CrossRef]
- Fontmorin, J.; Castillo, R.B.; Tang, W.; Sillanpää, M. Stability of 5, 5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef]
- Khan, A.; Wang, H.; Liu, Y.; Jawad, A.; Ifthikar, J.; Liao, Z.; Wang, T.; Chen, Z. Highly efficient α-Mn2O3@ α-MnO2-500 nanocomposite for peroxymonosulfate activation: Comprehensive investigation of manganese oxides. J. Mater. Chem. A 2018, 6, 1590–1600. [Google Scholar] [CrossRef]
- Ma, Q.; Pei, Y.; Dai, D.; Peng, X.; Cui, D.; Liu, C.; Yuan, L. Core-shell structured Mn2O3/MgO microsphere for removal of C.I. Basic Violet 3 from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 545, 188–196. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, T.; Wang, H.; Zhang, H.; Sun, Y.; Chen, L.; Song, S.; Li, L.; Shi, H. Oxygen-defective MnO2−x rattle-type microspheres mediated singlet oxygen oxidation of organics by peroxymonosulfate activation. Chem. Eng. J. 2020, 394, 124458. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Croué, J.-P. Catalytic Ozonation of Oxalate with a Cerium Supported Palladium Oxide: An Efficient Degradation Not Relying on Hydroxyl Radical Oxidation. Environ. Sci. Technol. 2011, 45, 9339–9346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, G.; Liu, H.; Qu, J. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. Int. Ed. 2019, 58, 8134–8138. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.M.; Dick, G.; Bargar, J.R.; Tebo, B.M. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). Proc. Natl. Acad. Sci. USA 2005, 102, 5558–5563. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Ran, R.; Wu, X.; Weng, D. Phase structures, morphologies, and NO catalytic oxidation activities of single-phase MnO2 catalysts. Appl. Catal. A Gen. 2016, 514, 24–34. [Google Scholar] [CrossRef]
- Tan, X.; Wan, Y.; Huang, Y.; He, C.; Zhang, Z.; He, Z.; Hu, L.; Zeng, J.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Dong, X.; Sun, Z.; Duan, X.; Ren, B.; Zheng, S.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Maitarad, P.; Shi, L.; Rungrotmongkol, T.; Li, H.; Zhang, J.; Cao, W. Morphology-dependent properties of MnO x/ZrO2–CeO2 nanostructures for the selective catalytic reduction of NO with NH3. J. Phys. Chem. C 2013, 117, 10502–10511. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W.; Cheng, X.; Li, W. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin. Chem. Eng. J. 2018, 343, 128–137. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Fu, D.; Deng, J.; Deng, L. Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep. Purif. Technol. 2017, 175, 47–57. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Fan, S.; Yang, T.; Zhou, Q. Facile template synthesis of dumbbell-like Mn2O3 with oxygen vacancies for efficient degradation of organic pollutants by activating peroxymonosulfate. Catal. Sci. Technol. 2020, 10, 864–875. [Google Scholar] [CrossRef]
- Lin, H.; Li, S.; Deng, B.; Tan, W.; Li, R.; Xu, Y.; Zhang, H. Degradation of bisphenol A by activating peroxymonosulfate with Mn0.6Zn0.4Fe2O4 fabricated from spent Zn-Mn alkaline batteries. Chem. Eng. J. 2019, 364, 541–551. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Mackeyev, Y.; Lee, G.-S.; Kim, H.-J.; Tachikawa, T.; Hong, S.; Lee, S.; Kim, J.; Wilson, L.J.; et al. Selective Oxidative Degradation of Organic Pollutants by Singlet Oxygen-Mediated Photosensitization: Tin Porphyrin versus C60 Aminofullerene Systems. Environ. Sci. Technol. 2012, 46, 9606–9613. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, P.; Tang, H. Visible-light photocatalytic degradation of bisphenol A on NaBiO3 nanosheets in a wide pH range: A synergistic effect between photocatalytic oxidation and chemical oxidation. Chem. Eng. J. 2016, 291, 149–160. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.-H. Reactivity and Transformation of Antibacterial N-Oxides in the Presence of Manganese Oxide. Environ. Sci. Technol. 2004, 39, 593–601. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, C.; Yan, X.; Chen, S.; Wang, C.; Luo, R.; Qi, J.; Sun, X.; Wang, L.; Li, J. Efficient Removal of Organic Pollutants by Metal–organic Framework Derived Co/C Yolk–Shell Nanoreactors: Size-Exclusion and Confinement Effect. Environ. Sci. Technol. 2020, 54, 10289–10300. [Google Scholar] [CrossRef]
- Lin, K.; Liu, W.; Gan, J. Oxidative Removal of Bisphenol A by manganese dioxide efficacy, products and pathway. Environ. Sci. Technol. 2009, 43, 3860–3864. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Zhang, S.; Liang, M.; Li, F.; Yuan, Y. Enhanced mineralization of bisphenol A by eco-friendly BiFeO3–MnO2 composite: Performance, mechanism and toxicity assessment. J. Hazard. Mater. 2020, 399, 122883. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.H.; Liang, H.; Zhou, G.; Wang, S. Supported cobalt catalysts by one-pot aqueous combustion synthesis for catalytic phenol degradation. J. Colloid Interface Sci. 2012, 394, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P.J. Optimization of the catalytic activity of a ZnCo2O4 catalyst in peroxymonosulfate activation for bisphenol A removal using response surface methodology. Chemosphere 2018, 212, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Dong, S.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2018, 647, 352–361. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, X.H.; Tam, N.F.-Y. Analysis and occurrence of typical endocrine-disrupting chemicals in three sewage treatment plants. Water Sci. Technol. 2010, 62, 2501–2509. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Ayten, N.; Olmez-Hanci, T. Photo-Fenton-like treatment of the commercially important H-acid: Process optimization by factorial design and effects of photocatalytic treatment on activated sludge inhibition. Appl. Catal. B Environ. 2010, 96, 208–217. [Google Scholar] [CrossRef]











| No. | Catalyst | BPA (mg/L) | PMS (mM) | Catalyst (g/L) | Removal Rate (%) | TOC Removal Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Mn0.8Fe2.2O4 | 9 | 0.4 | 0.1 | 100 (60 min) | 34 | [11] |
| 2 | CoMnAl | 10 | 0.24 | 0.02 | 100 (90 min) | 35 | [20] |
| 3 | MnOx/N-HPCS | 10 | 0.3 | 0.06 | 99 (120 min) | 39 | [22] |
| 4 | MnFe2O4 | 10 | 1.0 | 0.5 | 88 (90 min) | 51 | [19] |
| 5 | YS-Mn3O4 | 10 | 0.5 | 0.1 | 88 (60 min) | 58 | [23] |
| 6 | Mn/Fe3O4 | 23 | 2.0 | 0.2 | 100 (30 min) | 64 | [24] |
| 7 | Mn0.6Zn0.4Fe2O4 | 23 | 0.5 | 0.2 | 96 (60 min) | 70 | [75] |
| 8 | MnFeO | 10 | 0.3 | 0.1 | 100 (30 min) | 80 | [32] |
| 9 | Mn1.8Fe1.2O4 | 10 | 0.3 | 0.1 | 100 (30 min) | 80 | [32] |
| 10 | BiFeO3–MnO2 | 50 | 1.0 | 0.3 | 100 (30 min) | 85 | [81] |
| 11 | Mn2O3 | 10 | 0.1 | 0.2 | 100 (20 min) | 94 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Fu, W.; Hou, C.; Yang, Y.; Zhang, X. A Comprehensive Assessment of Catalytic Performances of Mn2O3 Nanoparticles for Peroxymonosulfate Activation during Bisphenol A Degradation. Catalysts 2021, 11, 993. https://doi.org/10.3390/catal11080993
Chen L, Fu W, Hou C, Yang Y, Zhang X. A Comprehensive Assessment of Catalytic Performances of Mn2O3 Nanoparticles for Peroxymonosulfate Activation during Bisphenol A Degradation. Catalysts. 2021; 11(8):993. https://doi.org/10.3390/catal11080993
Chicago/Turabian StyleChen, Li, Wanyi Fu, Congyu Hou, Yulong Yang, and Xihui Zhang. 2021. "A Comprehensive Assessment of Catalytic Performances of Mn2O3 Nanoparticles for Peroxymonosulfate Activation during Bisphenol A Degradation" Catalysts 11, no. 8: 993. https://doi.org/10.3390/catal11080993