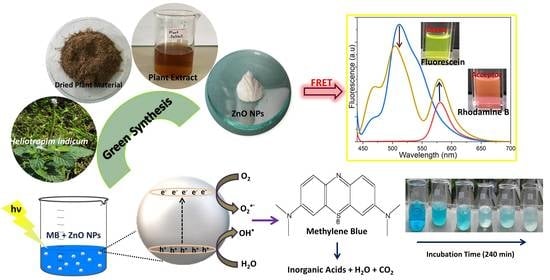
Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium indicum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis, Optimization of Synthesis Parameters, and Characterization of ZnO NPs
2.2. FTIR Spectroscopic Analysis of Green Synthesized ZnO NPs
2.3. Surface Morphology, Particle Size, and Nature of Biosynthesized NPs
2.4. Photocatalytic Activity of the Biosynthesized ZnO NPs
2.5. PL Activity of Synthesized ZnO NPs
2.6. FRET Ability of Biosynthesized ZnO NPs
3. Materials and Methodology
3.1. Chemicals and Reagents
3.2. Instruments
3.3. Collection of H. indicum and Preparation of Extracts
3.4. Optimization of Parameters for ZnO NPs Synthesis
3.5. Biosynthesis of ZnO NPs
3.6. Physical Characterization of ZnO NPs
3.7. Photocatalytic Activity of ZnO NPs
3.8. PL of the Synthesized ZnO NPs
3.9. FRET Activity of the Synthesized ZnO NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DI H2O | Deionized water |
| Flu | Fluorescein |
| FRET | Fluorescence resonance energy transfer |
| FTIR | Fourier transform infrared |
| H. indicum | Heliotropium indicum |
| MB | Methylene blue |
| MeOH | Methanol |
| MONPs | Metal oxide nanoparticles |
| NaOH | Sodium hydroxide |
| NPs | Nanoparticles |
| O2•− | Superoxide radicals |
| •OH | Hydroxyl radicals |
| PDI | Polydispersity index |
| PL | Photoluminescence |
| PS | Particle size |
| PSD | Particle size distribution |
| RhB | Rhodamine B |
| RT | Room temperature |
| SSA | Specific surface area |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TiO2 | Titanium dioxide |
| UV-Vis | Ultraviolet-Visible |
| XRD | X-ray diffraction |
| ZnO | Zinc oxide |
References
- Parra, M.R.; Haque, F.Z. Aqueous chemical route synthesis and the effect of calcination temperature on the structural and optical properties of ZnO nanoparticles. J. Mater. Res. Technol. 2014, 3, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Jamdagni, P.; Khatri, P.; Rana, J. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Kumar, S.V.; RajeshKumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process. Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Diallo, A.; Kaviyarasu, K.; Ndiaye, S.; Mothudi, B.M.; Ishaq, A.; Rajendran, V.; Maaza, M. Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem. Lett. Rev. 2018, 11, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Perera, K.M.K.G.; Kuruppu, K.A.S.S.; Chamara, A.M.R.; Thiripuranathar, G. Characterization of spherical Ag nanoparticles synthesized from the agricultural wastes of Garcinia mangostana and Nephelium lappaceum and their applications as a photo catalyzer and fluorescence quencher. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Kei, L.M.; Liang, W.H. Green synthesis of copper oxide (CuO) nanoparticles using banana peel extract and their photocatalytic activities. AIP Conf. Proc. 2017, 1828. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Yadav, L.S.R.; Lingaraju, K.; Prasad, B.D.; Kavitha, C.; Banuprakash, G.; Nagaraju, G. Synthesis of CeO2 nanoparticles: Photocatalytic and antibacterial activities. Eur. Phys. J. Plus 2017, 132. [Google Scholar] [CrossRef]
- Sreekanth, T.; Shim, J.-J.; Lee, Y.R. Degradation of organic pollutants by bio-inspired rectangular and hexagonal titanium dioxide nanostructures. J. Photochem. Photobiol. B Biol. 2017, 169, 90–95. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Crespo, J.D.S. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15. [Google Scholar] [CrossRef]
- Basavalingiah, K.R.; Harishkumar, S.; Nagaraju, G.; Rangappa, D. Highly porous, honeycomb like Ag–ZnO nanomaterials for enhanced photocatalytic and photoluminescence studies: Green synthesis using Azadirachta indica gum. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.A.; Ravikumar, C.; Nagaswarupa, H.; Purshotam, B.; Gonfa, B.; Murthy, H.A.; Sabir, F.K.; Tadesse, S. Evaluation of bi-functional applications of ZnO nanoparticles prepared by green and chemical methods. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Wijesinghe, U.; Thiripuranathar, G.; Iqbal, H.; Menaa, F. Biomimetic Synthesis, Characterization, and Evaluation of Fluorescence Resonance Energy Transfer, Photoluminescence, and Photocatalytic Activity of Zinc Oxide Nanoparticles. Sustainability 2021, 13, 2004. [Google Scholar] [CrossRef]
- Suresh, D.; Shobharani, R.; Nethravathi, P.; Kumar, M.P.; Bhushana, N.; Sharma, S. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 128–134. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Nithya, K.; Kalyanasundharam, S. Effect of chemically synthesis compared to biosynthesized ZnO nanoparticles using aqueous extract of C. halicacabum and their antibacterial activity. OpenNano 2019, 4. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Hussain, S.Z.; Huma, Z.-E.; Shahnaz, G.; Qureshi, O.S.; Khalid, Q.; Mirza, S.; Hussain, I.; Webster, T.J. Green synthesis of zinc oxide nanoparticles by Neem extract as multi-facet therapeutic agents. J. Drug Deliv. Sci. Technol. 2020, 59. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.; Rajanaika, H.; Nagabhushana, H.; Sharma, S. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, P.; Shruthi, G.; Suresh, D.; Nagabhushana, H.; Sharma, S. Garcinia xanthochymus mediated green synthesis of ZnO nanoparticles: Photoluminescence, photocatalytic and antioxidant activity studies. Ceram. Int. 2015, 41, 8680–8687. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2014, 5, 4993–5003. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Reddy, A.R.; Sujatha, C.; Reddy, K.V. Optimization of UV emission intensity of ZnO nanoparticles by changing the excitation wavelength. Mater. Lett. 2013, 99, 97–100. [Google Scholar] [CrossRef]
- Talakonda, P.R. Excitation-Intensity (EI) Effect on Photoluminescence of ZnO Materials with Various Morphologies. In Luminescence—An Outlook on the Phenomena and Their Applications; Thirumalai, J., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Ashrafi, M. Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nano. Chem. Res. 2016, 1, 79–86. [Google Scholar]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2017, 25, 10328–10339. [Google Scholar] [CrossRef]
- Adam, R.E.; Pozina, G.; Willander, M.; Nur, O. Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photon. Nanostructures Fundam. Appl. 2018, 32, 11–18. [Google Scholar] [CrossRef]
- Kuruppu, K.A.S.S.; Perera, K.M.K.G.; Chamara, A.M.R.; Thiripuranathar, G. Flower shaped ZnO—NPs; phytofabrication, photocatalytic, fluorescence quenching, and photoluminescence activities. Nano Express 2020, 1. [Google Scholar] [CrossRef]
- Saha, J.; Roy, A.D.; Dey, D.; Bhattacharjee, D.; Paul, P.K.; Das, R.; Hussain, S.A. Effect of Zinc oxide nanoparticle on Fluorescence Resonance Energy transfer between Fluorescein and Rhodamine 6G. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 110–116. [Google Scholar] [CrossRef]
- Saha, J.; Roy, A.D.; Dey, D.; Bhattacharjee, D.; Hussain, S.A. Role of quantum dot in designing FRET based sensors. Mater. Today Proc. 2018, 5, 2306–2313. [Google Scholar] [CrossRef]
- Sakr, M.E.M.; Kana, M.T.H.A.; Fattah, G.A. Fluorescence enhancement monitoring of pyrromethene laser dyes by metallic Ag nanoparticles. Luminescence 2014, 29, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Tovmachenko, O.G.; Graf, C.; Heuvel, D.J.V.D.; Van Blaaderen, A.; Gerritsen, H.C. Fluorescence Enhancement by Metal-Core/Silica-Shell Nanoparticles. Adv. Mater. 2006, 18, 91–95. [Google Scholar] [CrossRef]
- Dey, D.; Bhattacharjee, D.; Chakraborty, S.; Hussain, S.A. Development of hard water sensor using fluorescence resonance energy transfer. Sens. Actuators B Chem. 2013, 184, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Y.; Xiong, H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, E.P. Fluorescence Detection of Zinc Oxide Nanoparticles in Water Contamination Analysis based on Surface Reactivity with Porphyrin. AIMS Environ. Sci. 2018, 5, 67–77. [Google Scholar] [CrossRef]
- Yue, Q.; Cheng, J.; Li, G.; Zhang, K.; Zhai, Y.; Wang, L.; Liu, J. Fluorescence Property of ZnO Nanoparticles and the Interaction with Bromothymol Blue. J. Fluoresc. 2010, 21, 1131–1135. [Google Scholar] [CrossRef]
- Kalele, S.; Deshpande, A.C.; Singh, S.B.; Kulkarni, S.K. Tuning luminescence intensity of RHO6G dye using silver nanoparticles. Bull. Mater. Sci. 2008, 31, 541–544. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Gnanasangeetha, D.; Thambavani, D. Biogenic Production of Zinc Oxide Nanoparticles Using Acalypha Indica. J. Chem. Biol. Phys. Sci. 2014, 4, 238–246. [Google Scholar]
- Osman, D.; Mustafa, M. Synthesis and Characterization of Zinc Oxide Nanoparticles using Zinc Acetate Dihydrate and Sodium Hydroxide. J. Nanosci. Nanoeng. 2015, 1, 248–251. [Google Scholar]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Safawo, T.; Sandeep, B.; Pola, S.; Tadesse, A. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano 2018, 3, 56–63. [Google Scholar] [CrossRef]
- Blažeka, D.; Car, J.; Klobučar, N.; Jurov, A.; Zavašnik, J.; Jagodar, A.; Kovačević, E.; Krstulović, N. Photodegradation of Methylene Blue and Rhodamine B Using Laser-Synthesized ZnO Nanoparticles. Materials 2020, 13, 4357. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.K.; Murthy, P.N. Studies on Wound Healing Activity of Heliotropium indicum Linn. Leaves on Rats. ISRN Pharmacol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adjagba, M.; Awede, B.; Osseni, R.; Dougnon, G.; Dosseh, W.; Lagnika, L.; Darboux, R.; Laleye, A. Effects of crude aqueous extracts of Heliotropium indicum Linn (Boraginaceae) on blood pressure in Wistar rats. Int. J. Pharmacol. Res. 2017, 7, 165–169. [Google Scholar] [CrossRef]
- Mourin, N.A.; Sharmin, T.; Chowdhury, S.R.; Islam, F.; Rahman, M.S.; Rashid, M.A. Evaluation of bioactivities of Heliotropium indicum, a medicinal plant of Bangladesh. Pharma. Innov. 2013, 2, 217–221. [Google Scholar]
- Jayachitra, J.; Bharathim, M. In vitro studies on phytochemical analysis and antioxidant activity of Heliotropium indicum linn. Int. J. Res. Pharmacol. Pharmacother. 2016, 5, 108–114. [Google Scholar]
- Goyal, N.; Sharma, S. Bioactive phytoconstituents and plant extracts from genus Heliotropium. Int. J. Green Pharm. 2014, 8. [Google Scholar] [CrossRef]
- Roy, A.; Chowdhury, G. Pharmacological Activities of Indian Heliotrope (Heliotropium Indicum L.): A Review. J. Pharmacogn. Phytochem. 2015, 4, 101–104. [Google Scholar]
- Wang, Z.; Huang, B.; Liu, X.; Qin, X.; Zhang, X.; Wei, J.; Wang, P.; Yao, S.; Zhang, Q.; Jing, X. Photoluminescence studies from ZnO nanorod arrays synthesized by hydrothermal method with polyvinyl alcohol as surfactant. Mater. Lett. 2008, 62, 2637–2639. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Hahn, Y. ZnO Nanoparticles: Growth, Properties, and Applications. In Metal Oxide Nanostructures and Their Applications; American Scientific Publishers: Stevenson Ranch, CA, USA, 2010; Volume 5. [Google Scholar]
- Manokari, M.; Shekhawat, M.S. Green synthesis of zinc oxide nanoparticles using whole plant extracts of Cassia tora L. and their characterization. J. Sci. Achiev. 2017, 2, 10–16. [Google Scholar]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Gnanasangeetha, D.; Saralathambavani, D. One Pot Synthesis of Zinc Oxide Nanoparticles via Chemical and Green Method. Res. J. Mater. Sci. 2013, 1, 1–8. [Google Scholar]
- Koshy, J.; Samuel, M.S.; Chandran, A.; George, K.C. Optical Properties of CuO Nanoparticles. AIP Conf. Proc. 2011, 1391, 576–578. [Google Scholar] [CrossRef]
- Shah, R.K.; Boruah, F.; Parween, N. Synthesis and Characterization of ZnO Nanoparticles using Leaf Extract of Camellia sinesis and Evaluation of their Antimicrobial Efficacy. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 444–450. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; RajeshKumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M. Sageretia thea (Osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications. Nanomedicine 2017, 12, 1767–1789. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Lingaraju, K.; Naika, H.R.; Manjunath, K.; Basavaraj, R.B.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016, 6, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Nath, B.; Barbhuiya, T.F. Studies on the density and surface area of nanoparticles from Camellia sinensis, A natural source. J. Chem. Pharm. Res. 2014, 6, 608–610. [Google Scholar]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Azab, W.I.M.E.; Ali, H.R.; Mansour, M.S.M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Saraswathi, S.; Tatsugi, J.; Shin, P.-K.; Santhakumar, K. Facile biosynthesis, characterization, and solar assisted photocatalytic effect of ZnO nanoparticles mediated by leaves of L. speciosa. J. Photochem. Photobiol. B Biol. 2017, 167, 89–98. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, R.; Gillani, S.S.A.; Tahir, M.B.; Shakil, M.; Iqbal, T.; Abdellahi, M.O. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, Z.; Fazal, A.; Irfan, M. Use of Vegetable Waste Extracts for Controlling Microstructure of CuO Nanoparticles: Green Synthesis, Characterization, and Photocatalytic Applications. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Sinha, T. A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Manjunath, K.; Nagaraju, G.; Suresh, D.; Bhushana, N. Rauvolfia serpentina-Mediated Green Synthesis of CuO Nanoparticles and Its Multidisciplinary Studies. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1134–1140. [Google Scholar] [CrossRef]











| Plant Name | Photo-Nanocatalyst | Dye | Source of Irradiation | Degradation Efficiency (%) and Time | Reference |
|---|---|---|---|---|---|
| Tephrosia purpurea | ZnO NPs | Methylene blue (MB) | Sunlight | 98.86 in 240 min | [15] |
| Phoenix dactylifera | ZnO NPs | MB | UV light | 90.5 in 180 min | [23] |
| Carissa edulis | ZnO NPs | Congo red | Photoreactor | 97 in 135 min | [70] |
| Lagerstroemia speciosa | ZnO NPs | Methyl orange (MO) | Sunlight | 93.5 in 120 min | [73] |
| Eucalyptus globulus | ZnO NPs | MB and MO | UV light | 98.3, 50 min and 97 in 60 min | [72] |
| Corriandrum sativum | ZnO NPs | Anthracene | UV light | 96 in 240 min | [71] |
| Syzygium cumini | ZnO NPs | Rhodamine B | UV light | 98 in 110 min | [74] |
| Rhizoma Coptidis | TiO2 NPs | MB and Malachite green | UV light | 71 and 78 in 60 min | [11] |
| Parthenium hysterophorus | TiO2 NPs | MB, MO, Crystal violet and Alizarin red | Visible light | 92.5, 81.5, 79.7 and 77.3 in 360 min | [30] |
| Sugar cane juice | CeO2 NPs | MB | UV light Sunlight | 94 and 86 in 180 min | [10] |
| Sugar cane juice | SnO2 NPs | MB and Rose Bengal | sunlight | 99.3 and 96.8 in 300 min | [76] |
| Cynometra ramiflora | FeO NPs | MB | Sunlight | 98 in 240 min | [77] |
| Banana peel | CuO NPs | Congo red | Sunlight | 88 in | [8] |
| Rauvolfia serpentina | CuO NPs | Trypan blue | Sunlight and UV light | >90 in 180 min | [78] |
| Cauliflower waste, Potatoe peel and Pea peel | CuO NPs | MB | Irradiation | 96.28 87.37 79.11 in 120 min | [75] |
| Aspalathus linearis | NiO NPs | MB | Sunlight and UV light | >50 in 120 min | [6] |
| ZnO NPs | Half-Lifetime (min) | Degradation Efficiency (%) |
|---|---|---|
| HIS | 97.30 | 95.0 |
| HIL | 135.00 | 93.1 |
| HIF | 100.00 | 95.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesinghe, U.; Thiripuranathar, G.; Menaa, F.; Iqbal, H.; Razzaq, A.; Almukhlifi, H. Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium indicum. Catalysts 2021, 11, 831. https://doi.org/10.3390/catal11070831
Wijesinghe U, Thiripuranathar G, Menaa F, Iqbal H, Razzaq A, Almukhlifi H. Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium indicum. Catalysts. 2021; 11(7):831. https://doi.org/10.3390/catal11070831
Chicago/Turabian StyleWijesinghe, Udari, Gobika Thiripuranathar, Farid Menaa, Haroon Iqbal, Anam Razzaq, and Hanadi Almukhlifi. 2021. "Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium indicum" Catalysts 11, no. 7: 831. https://doi.org/10.3390/catal11070831