Nanoscale Assembly of BiVO4/CdS/CoOx Core–Shell Heterojunction for Enhanced Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterization of BiVO4-Based Photoanodes
2.2. Photoelectrochemical Water Splitting Investigation
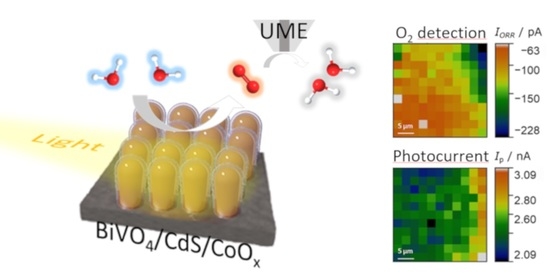
2.3. Scanning Photoelectrochemical Microscope Measurements
2.4. Electrochemical Impedance Spectroscopy and Intensity Modulated Photocurrent Spectroscopy Measurements
3. Experimental
3.1. Materials
3.2. BiVO4 Photoanodes Preparation
3.3. Preparation of BiVO4/CdS and BiVO4/CdS/CoOx Heterostructures
3.4. Photoelectrochemical Measurements
3.5. Scanning Photoelectrochemical Microscopy Measurements
3.6. Physical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Gibson, T.L.; Kelly, N.A. Optimization of solar powered hydrogen production using photovoltaic electrolysis devices. Int. J. Hydrogen Energy 2008, 33, 5931–5940. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A. 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. BiVO4-Based Heterostructured photocatalysts for solar water splitting: A review. Energy Environ. Focus 2014, 3, 339–353. [Google Scholar] [CrossRef]
- Yalavarthi, R.; Zbořil, R.; Schmuki, P.; Naldoni, A.; Kment, Š. Elucidating the role of surface states of BiVO4 with Mo doping and a CoOOH co-catalyst for photoelectrochemical water splitting. J. Power Sources 2021, 483, 229080. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.-W.; Jo, Y.H.; Abdi, F.F.; Lee, Y.H.; van de Krol, R.; Lee, J.S. Hetero-type dual photoanodes for unbiased solar water splitting with extended light harvesting. Nat. Commun. 2016, 7, 13380. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Tsubota, T.; Mooij, L.P.A.; van de Krol, R. Highly improved quantum efficiencies for thin film BiVO4 photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. [Google Scholar] [CrossRef]
- Friedrich, D.; Kressman, S.; Strub, H.; Artero, V.; Laberty-Robert, C.; Hillard, S. Solar-water-splitting BiVO4 thin-film photoanodes prepared by using a sol–gel dip-coating technique. ChemPhotoChem 2017, 1, 273–280. [Google Scholar] [CrossRef]
- Chen, L.; Alarcón-Lladó, E.; Hettick, M.; Sharp, I.D.; Lin, Y.; Javey, A.; Ager, J.W. Reactive sputtering of bismuth vanadate photoanodes for solar water splitting. J. Phys. Chem. C 2013, 117, 21635–21642. [Google Scholar] [CrossRef] [Green Version]
- Murcia-López, S.; Fàbrega, C.; Monllor-Satoca, D.; Hernández-Alonso, M.D.; Penelas-Pérez, G.; Morata, A.; Morante, J.R.; Andreu, T. Tailoring multilayered BiVO4 photoanodes by pulsed laser deposition for water splitting. ACS Appl. Mater. Inter. 2016, 8, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Kölbach, M.; Harbauer, K.; Ellmer, K. Elucidating the pulsed laser deposition process of BiVO4 photoelectrodes for solar water splitting. J. Phys. Chem. C 2020, 124, 4438–4447. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Arai, T.; Sugita, T.; Abe, R.; Yanagida, M.; Oi, T.; Iwasaki, Y.; Abe, Y.; Sugihara, H. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment. J. Phys. Chem. B 2006, 110, 11352–11360. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Mueller, A.H.; McCleskey, T.M.; Burrell, A.K.; Bauer, E.; Jia, Q.X. Structural and photoelectrochemical properties of BiVO4 thin-films. J. Phys. Chem. C 2008, 112, 6099–6102. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chou, L.; Bi, Y. Tuning surface electronegativity of BiVO4 photoanodes toward highperformance water splitting. Appl. Catal. B Environ. 2020, 262, 118267. [Google Scholar] [CrossRef]
- Seabold, J.A.; Zhu, K.; Neale, N.R. Efficient solar photoelectrolysis by nanoporous Mo:BiVO4 through controlled electron transport. Phys. Chem. Chem. Phys. 2013, 16, 1121–1131. [Google Scholar] [CrossRef]
- Jua, S.; Seokb, H.-J.; Juna, J.; Huha, D.; Sona, S.; Kima, K.; Kima, W.; Baeka, S.; Kimb, H.-K.; Leea, H. Fully blossomed WO3/BiVO4 structure obtained via active facet engineering of patterned FTO for highly efficient water splitting. Appl. Catal. B Environ. 2020, 263, 118362. [Google Scholar] [CrossRef]
- She, H.; Yue, P.; Ma, X.; Huang, J.; Wang, L.; Wang, Q. Fabrication of BiVO4 photoanode cocatalyzed with NiCo-layered double hydroxide for enhanced photoactivity of water oxidation. Appl. Catal. B Environ. 2020, 263, 118280. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, K.; Huang, J.; Wang, L.; Zhang, M.; Bai, B.; Liu, H.; Wang, Q. Preparation of heterometallic CoNi-MOFs-modified BiVO4: A steady photoanode for improved performance in photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 266, 118513. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Kuang, Y.; Zhao, J.; Liu, M.; Iwase, A.; Jia, Q.; Nishiyama, H.; Minegishi, T.; Nakabayashi, M.; et al. Surface modification of CoOx loaded BiVO4 photoanodes with ultrathin p-Type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 2015, 137, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, T.; Liu, Z.; Qi, L. A cobalt silicate modified BiVO4 photoanode for efficient solar water oxidation. Appl. Catal. B-Environ. 2020, 277, 119189. [Google Scholar] [CrossRef]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef]
- Shi, X.; Choi, I.Y.; Zhang, K.; Kwon, J.; Kim, D.Y.; Lee, J.K.; Oh, S.H.; Kim, J.K.; Park, J.H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures. Nat. Commun. 2014, 5, 4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentová, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zbořil, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Li, H.; Xia, Z.; Chen, J.; Lei, L.; Xing, J. Constructing ternary CdS/reduced graphene oxide/TiO2 nanotube arrays hybrids for enhanced visible-light-driven photoelectrochemical and photocatalytic activity. Appl. Catal. B Environ. 2015, 168–169, 105–113. [Google Scholar] [CrossRef]
- Rambabu, Y.; Dhua, S.; Jaiswal, M.; Roy, S.C. High photoelectrochemical performance of reduced graphene oxide wrapped, CdS functionalized, TiO2 multi-leg nanotubes. Nanotechnology 2020, 31, 275701. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Gopalakrishnan, A.; Borse, P.H. Efficiency and stability aspects of CdS photoanode for solar hydrogen generation technology. J. Phys. Conf. Ser. 2016, 755, 012006. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef]
- Han, B.; Liu, S.; Xu, Y.-J.; Tang, Z.-R. 1D CdS nanowire–2D BiVO4 nanosheet heterostructures toward photocatalytic selective fine-chemical synthesis. RSC Adv. 2015, 5, 16476–16483. [Google Scholar] [CrossRef]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Constructing BiVO4-Au@CdS photocatalyst with energic charge-carrier-separation capacity derived from facet induction and Z-scheme bridge for degradation of organic pollutants. Appl. Catal. B Environ. 2018, 227, 258–265. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Wang, L.; Han, M.; Zhang, M.; Wang, H.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl. Catal. B Environ. 2017, 206, 501–509. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; Chang, S.; Tian, B.; Zhang, J. Z-scheme CdS–Au–BiVO4 with enhanced photocatalytic activity for organic contaminant decomposition. Catal. Sci. Technol. 2017, 7, 124–132. [Google Scholar] [CrossRef]
- Meissner, D.; Memming, R.; Kastening, B.; Bahnemann, D. Fundamental problems of water splitting at cadmium sulfide. Chem. Phys. Lett. 1986, 127, 419–423. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Yang, C.; Cheng, H.; Jiao, S.; Zhu, H. Cobalt-bilayer catalyst decorated Ta3N5 nanorod arrays as integrated electrodes for photoelectrochemical water oxidation. Energy Environ. Sci. 2013, 6, 3322–3330. [Google Scholar] [CrossRef]
- Yalavarthi, R.; Henrotte, O.; Minguzzi, A.; Ghigna, P.; Grave, D.A.; Naldoni, A. In situ characterizations of photoelectrochemical cells for solar fuels and chemicals. MRS Energy Sustain. 2020, 7. [Google Scholar] [CrossRef]
- Moniruddin, M.; Oppong, E.; Stewart, D.; McCleese, C.; Roy, A.; Warzywoda, J.; Nuraje, N. Designing CdS-based ternary heterostructures consisting of Co-metal and CoOx cocatalysts for photocatalytic H2 evolution under visible light. Inorg. Chem. 2019, 58, 12325–12333. [Google Scholar] [CrossRef]
- Jina, S.; Maa, X.; Pana, J.; Zhub, C.; Sajic, S.E.; Hua, J.; Xua, X.; Sunb, L.; Yinc, Z. Oxygen vacancies activating surface reactivity to favor charge separation and transfer in nanoporous BiVO4 photoanodes. Appl. Catal. B Environ. 2021, 281, 119477. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, S.; Shi, Y.; Liu, X.; Wu, Z.; Jiang, Q.; Zhou, T.; Liu, N.; Hu, J. Construction of CdS/CoOx core-shell nanorods for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 234, 109–116. [Google Scholar] [CrossRef]
- Son, J.S.; Lee, J.-S.; Shevchenko, E.V.; Talapin, D.V. Magnet-in-the-semiconductor nanomaterials: High electron Mobility in all-inorganic arrays of FePt/CdSe and FePt/CdS core–shell heterostructures. J. Phys. Chem. Lett. 2013, 4, 1918–1923. [Google Scholar] [CrossRef]
- James, P.; Casillas, N.; Smyrl, W.H. Simultaneous scanning electrochemical and photoelectrochemical microscopy by use of a metallized optical fiber. J. Electrochem. Soc. 1996, 143, 3853. [Google Scholar] [CrossRef]
- Govindaraju, G.V.; Wheeler, G.P.; Lee, D.; Choi, K.-S. Methods for electrochemical synthesis and photoelectrochemical characterization for photoelectrodes. Chem. Mater. 2017, 29, 355–370. [Google Scholar] [CrossRef]
- Zhao, F.; Plumeré, N.; Nowaczyk, M.M.; Ruff, A.; Schuhmann, W.; Conzuelo, F. Interrogation of a PS1-Based Photocathode by Means of scanning photoelectrochemical microscopy. Small 2017, 13, 1604093. [Google Scholar] [CrossRef]








| Sample | RS (Ω) | RSC (Ω) | Rct (Ω) | CPE2 (µF) |
|---|---|---|---|---|
| BiVO4 | 24.43 ± 1.2 | 116 ± 14 | 992 ± 15 | 87.9 ± 0.8 |
| BiVO4/CdS | 19.07 ± 1.6 | 64 ± 17 | 390 ± 12 | 170 ± 1.9 |
| BiVO4/CdS/CoOx | 12.61 ± 0.7 | 46 ± 2 | 305 ± 6 | 203 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmentova, H.; Henrotte, O.; Yalavarthi, R.; Haensch, M.; Heinemann, C.; Zbořil, R.; Schmuki, P.; Kment, Š.; Naldoni, A. Nanoscale Assembly of BiVO4/CdS/CoOx Core–Shell Heterojunction for Enhanced Photoelectrochemical Water Splitting. Catalysts 2021, 11, 682. https://doi.org/10.3390/catal11060682
Kmentova H, Henrotte O, Yalavarthi R, Haensch M, Heinemann C, Zbořil R, Schmuki P, Kment Š, Naldoni A. Nanoscale Assembly of BiVO4/CdS/CoOx Core–Shell Heterojunction for Enhanced Photoelectrochemical Water Splitting. Catalysts. 2021; 11(6):682. https://doi.org/10.3390/catal11060682
Chicago/Turabian StyleKmentova, Hana, Olivier Henrotte, Rambabu Yalavarthi, Mareike Haensch, Christian Heinemann, Radek Zbořil, Patrik Schmuki, Štěpán Kment, and Alberto Naldoni. 2021. "Nanoscale Assembly of BiVO4/CdS/CoOx Core–Shell Heterojunction for Enhanced Photoelectrochemical Water Splitting" Catalysts 11, no. 6: 682. https://doi.org/10.3390/catal11060682