Copper Tricomponent Catalysts Application for Hydrogen Production from Ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of the Catalysts
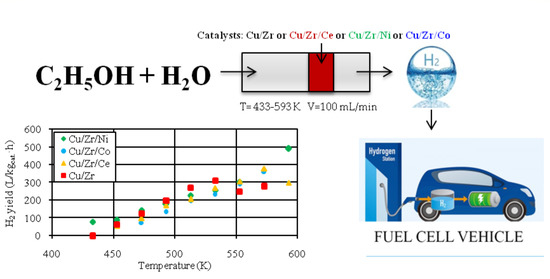
2.2. Catalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238–106249. [Google Scholar] [CrossRef]
- Gonzalez-Gil, R.; Chamorro-Burgos, I.; Herrera, C.; Larrubia, M.A.; Laborde, M.; Marino, F.; Alemany, L.J. Production of hydrogen by catalytic steam reforming of oxygenated model compounds on Ni-modified supported catalysts. Simulation and experimental study. Int. J. Hydrogen Energy 2015, 40, 11217–11227. [Google Scholar] [CrossRef]
- Madej-Lachowska, M.; Kulawska, M.; Słoczyński, J. Methanol as a high purity hydrogen source for fuel cells: A brief review of catalysts and rate expressions. Chem. Proc. Eng. 2017, 38, 147–162. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 80–93. [Google Scholar]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of hydrogen production using chemical-looping technology. Renew. Sust. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Jia, P.; Dong, D.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Hu, X. Ethanol steam reforming over cobalt catalysts: Effect of a range of additives on the catalytic behaviors. J. Energy Inst. 2020, 93, 165–184. [Google Scholar] [CrossRef]
- Vizcaino, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu–Ni supported catalysts. Int. J. Hydrogen Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Snytnikov, P.V.; Badmaev, S.D.; Volkova, G.G.; Potemkin, D.I.; Zyryanova, M.M.; Belyaev, V.D. Catalysts for hydrogen production in a multifuel processor by methanol, dimethyl ether and bioethanol steam reforming for fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 16388–16396. [Google Scholar] [CrossRef]
- Moraes, T.S.; Borges, L.E.P.; Farrauto, R.; Noronha, F.B. Steam reforming of ethanol on Rh/SiCeO2washcoated monolith catalyst: Stable catalyst performance. Int. J. Hydrogen Energy 2018, 43, 115–126. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. H2 Production for MC Fuel Cell by Steam Reforming of Ethanol Over MgO Supported Pd, Rh, Ni and Co Catalysts. Appl. Catal. B Environ. 2014, 145, 73–84. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, J.; Yu, J.; Yang, X.; Sheng, X.; Xu, H.; Sun, C.; Shen, W.; Goldbach, A. Efficient H2 production via membrane-assisted ethanol steam reforming over Ir/CeO2 catalyst. Int. J. Hydrogen Energy 2019, 44, 24733–24745. [Google Scholar] [CrossRef]
- Morales, M.; Segarra, M. Steam reforming and oxidative steam reforming of ethanol over La0.6Sr0.4CoO3−δ perovskite as catalyst precursor for hydrogen production. Appl. Catal. A Gen. 2015, 502, 305–311. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.; Yue, Y.; Liu, G.; Liu, Y. Nano bimetallic alloy of Ni-Co obtained fromLaCoxNi1−xO3 and its catalytic performance for steam reforming of ethanol. RSC Adv. 2015, 5, 16837–16916. [Google Scholar]
- Moraes, T.S.; Neto, R.C.R.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Ladas, S. Thestudy of the performance of PtNi/CeO2–nanocube catalysts for low temperaturesteam reforming of ethanol. Catal. Today 2015, 242, 35–49. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Lee, C.-L.; Ho, K.-F.; Huang, H.-H.; Yu, S.-W.; Wang, C.-B. Catalytic performance of Pt-promoted cobalt-based catalysts for the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 5653–5662. [Google Scholar] [CrossRef]
- Wang, F.; Cai, W.; Provendier, H.; Schuurman, Y.; Descorme, C.; Mirodatos, C. Hydrogen production from ethanol steam reforming over Ir/CeO2 catalysts: Enhanced stability by PrOx promotion. Int. J. Hydrogen Energy 2011, 36, 13566–13574. [Google Scholar] [CrossRef]
- Hamryszak, Ł.; Madej–Lachowska, M.; Kulawska, M.; Ruggiero-Mikołajczyk, M.; Samson, K.; Śliwa, M. Investigation on binary copper-based catalysts used in the ethanol steam reforming process. React. Kinet. Catal. Mech. 2020, 130, 727–739. [Google Scholar] [CrossRef]
- Fajardo, H.V.; Longo, E.; Mezalira, D.; Nuernberg, G.; Almerindo, G.; Collasiol, A.; Probst, L.F.D.; Garcia, I.T.S.; Carreño, N.L.V. Influence of support on catalytic behavior of nickel catalysts in the steam reforming of ethanol for hydrogen production. Environ. Chem. Lett. 2010, 8, 79–85. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts. Catal. Today 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by reformation of biomass-derived ethanol. Catal. Today 2002, 75, 145–155. [Google Scholar] [CrossRef]
- Rossetti, I.; Lasso, J.; Finocchio, E.; Ramis, G.; Nichele, V.; Signoretto, M.; Di Michele, A. TiO2-supported catalysts for the steam reforming of ethanol. Appl. Catal. A Gen. 2014, 477, 42–53. [Google Scholar] [CrossRef]
- Pinton, N.; Vidal, M.V.; Signoretto, M.; Martínez-Arias, A.; Cortés Corberán, V. Ethanol steam reforming on nanostructured catalysts of Ni, Co and CeO2: Influence of synthesis method on activity, deactivation and regenerability. Catal. Today 2017, 296, 135–143. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Zhang, C.; Wang, S.; Ma, X.; Gong, J. Steam reforming of ethanol over Ni/ZrO2 catalysts: Effect of support on product distribution. Int. J. Hydrogen Energy 2012, 37, 2940–2949. [Google Scholar] [CrossRef]
- Bergamaschi, V.S.; Carvalho, F.M.S.; Rodrigues, C.; Fernandes, D.B. Preparation and evaluation of zirconia microspheres as inorganic exchanger in adsorption of copper and nickel ions and as catalyst in hydrogen production from bioethanol. Chem. Eng. J. 2005, 112, 153–158. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Madej-Lachowska, M.; Moroz, H.; Wyżgoł, H.; Hamryszak, Ł. The investigation of activity the bimetallic catalysts based on nickel oxide, cobalt oxide, cerium oxide in ethanol steam reforming (ESR). In Research Papers of the Institute of Chemical Engineering; Polish Academy of Sciences: Warsaw, Poland, 2017; Volume 21, pp. 99–117. [Google Scholar]
- Batista, M.S.; Santos, R.K.S.; Assaf, E.M.; Assaf, J.M.; Ticianelli, E.A. High efficiency steam reforming of ethanol by cobalt-based catalysts. J. Power Sources 2004, 134, 27–32. [Google Scholar] [CrossRef]
- Augusto, B.L.; Ribeiro, M.C.; Aires, F.J.C.S.; da Silva, V.T.; Noronha, F.B. Hydrogen production by the steam reforming of ethanol over cobalt catalysts supported on different carbon nanostructures. Catal. Today 2020, 344, 66–74. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Hydrogen production by steam reforming of ethanol over Co/CeO2 catalysts: Effect of cobalt content. J. Energy Inst. 2019, 92, 222–238. [Google Scholar] [CrossRef]
- Kulawska, M.; Madej-Lachowska, M.; Hamryszak, Ł.; Moroz, H.; Wyżgoł, H. Application of copper catalyst to the hydrogen production by steam reforming of ethanol. Przem. Chem. 2019, 98, 1992–1995. [Google Scholar]
- de Lima, S.M.; Silva, A.M.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V. Ethanol decomposition and steam reforming of ethanol over CeZrO2 and Pt/CeZrO2 catalyst: Reaction mechanism and deactivation. Appl. Catal. A Gen. 2009, 352, 95–113. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Tasnadi-Asztalos, Z.; Imre-Lucaci, A.; Katona, G.; Lazar, M.D. Hydrogen production by ethanol steam reforming on nickel catalysts: Effect ofsupport modification by CeO2 and La2O3. Fuel 2015, 147, 260–268. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Arandia, A.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Reproducible performance of a Ni/La2O3–αAl2O3 catalyst in ethanol steam reforming under reaction–regeneration cycles. Fuel Process. Technol. 2016, 152, 215–222. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, L.; Xu, J.; Zheng, Q.; Pan, L.; Zou, J.; Zhang, X.; Li, G. Boosting hydrogen production from steam reforming of ethanol on nickel by lanthanum doped ceria. Appl. Catal. B Environ. 2021, 286, 119884–119897. [Google Scholar] [CrossRef]
- Kim, D.; Kwak, B.S.; Park, N.-K.; Han, G.B.; Kang, M. Dynamic hydrogen production from ethanol steam-reforming reaction on NixMoy/SBA-15 catalytic system. Int. J. Energy Res. 2015, 39, 279–292. [Google Scholar] [CrossRef]
- Barroso, M.N.; Gomez, M.F.; Arrua, L.A.; Abello, M.C. Hydrogen production by ethanol reforming over NiZnAl catalysts. Appl. Catal. A Gen. 2006, 304, 116–123. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; da Costa, L.O.O.; Ribeiro, M.C.; Rabelo-Neto, R.C.; Mattos, L.V.; Venugopal, A.; Noronh, F.B. Effect of Zn addition on the performance of Ni/Al2O3 catalyst for steam reforming of ethanol. Appl. Catal. A Gen. 2016, 519, 85–98. [Google Scholar] [CrossRef]
- Fang, W.; Paul, S.; Capron, M.; Biradar, A.V.; Umbarkar, S.B.; Dongare, M.K. Highlyloaded well dispersed stable Ni species in NiXMg2AlOYnanocomposites: Applicationto hydrogen production from bioethanol. Appl. Catal. B Environ. 2015, 166, 485–496. [Google Scholar] [CrossRef]
- Sohrabi, S.; Irankhah, A. Synthesis, characterization, and catalytic activity of Ni/CeMnO2 catalysts promoted by copper, cobalt, potassium and iron for ethanol steam reforming. Int. J. Hydrogen Energy. 2021, 46, 12846–12856. [Google Scholar] [CrossRef]
- Das, N.K.; Dalai, A.K.; Ranganathan, R. Hydrogen Yield from Low Temperature Steam Reforming of Ethanol. Can. J. Chem. Eng. 2007, 85, 92–100. [Google Scholar] [CrossRef]
- Śliwa, M.; Samson, K. Steam reforming of ethanol over copper-zirconiabased catalysts doped with Mn, Ni, Ga. Int. J. Hydrogen Energy 2021, 46, 555–564. [Google Scholar] [CrossRef]
- Dancini-Pontes, I.; DeSouza, M.; Silva, F.A.; Scaliante, M.H.N.O.; Alonso, C.G.; Bianchi, G.S.; Neto, A.M.; Pereira, G.M.; Fernandes-Machado, N.R.C. Influence of the CeO2 and Nb2O5 supports and the inert gas in ethanol steamreforming for H2 production. Chem. Eng. J. 2015, 273, 66–74. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lin, S.D. Effects of the pretreatment of CuNi/SiO2 on ethanol steam reforming: Influence of bimetal morphology. Appl. Catal. B Environ. 2014, 148–149, 509–519. [Google Scholar] [CrossRef]
- Donga, X.-F.; Zoub, H.-B.; Lin, W.-M. Effect of preparation conditions of CuO-CeO2-ZrO2 catalyst on CO removal from hydrogen-rich gas. Int. J. Hydrogen Energy 2006, 31, 2337–2344. [Google Scholar] [CrossRef]
- Ziółek, M.; Nowak, I. Kataliza Heterogeniczna–Wybrane Zagadnienia; Wydawnictwo Naukowe UAM: Poznań, Poland, 1999. [Google Scholar]
- Bahari, M.B.; Phuc, N.H.H.; Abdullah, B.; Alenazey, F.; Vo, D.-V.N. Ethanol dry reforming for syngas production over Ce-promoted Ni/Al2O3 catalyst. J. Environ. Chem. Eng. 2016, 4, 4830–4838. [Google Scholar] [CrossRef] [Green Version]
- Basahel, S.N.; Mokhtar, M.; Alsharaeh, E.H.; Ali, T.T.; Mahmoud, H.A.; Narasimharao, K. Physico-Chemical and Catalytic Properties of Mesoporous CuO-ZrO2 Catalysts. Catalysts 2016, 6, 57–77. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Li, F.; Zhan, H.J.; Zhao, N.; Xiao, F.K.; Wei, W.; Zhong, L.S.; Wang, H.; Sun, Y.H. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Di Cosimo, J.I. Acid–base properties and active site requirements for elimination reactions on alkali-promoted MgO catalysts. Catal. Today 2000, 63, 53–62. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Homogeneous precipitation synthesis of CuO–ZrO2–CeO2–Al2O3nanocatalyst used in hydrogen production via methanol steamreforming for fuel cell applications. Energy Convers. Manage. 2014, 87, 928–937. [Google Scholar] [CrossRef]
- Wang, G.; Zuo, Y.; Han, M.; Wang, J. Copper crystallite size and methanol synthesis catalytic property of Cu-based catalysts promoted by Al, Zr and Mn. Reac. Kinet. Mech. Cat. 2010, 101, 443–454. [Google Scholar] [CrossRef]
- Mastalir, A.; Frank, B.; Szizybalski, A.; Soerijanto, H.; Deshpande, A.; Niederberger, M.; Schomäcker, R.; Schlögl, R.; Ressler, T. Steam reforming of methanol over Cu/ZrO2/CeO2 catalysts: A kinetic study. J. Catal. 2005, 230, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Binet, C.; Daturi, M.; Lavalley, J.C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Ebiad, M.A.; Abd El-Hafiz, D.R.; Elsalamony, R.A.; Mohamed, L.S. Ni supported high surface area CeO2–ZrO2 catalysts for hydrogen production from ethanol steam reforming. RSC Adv. 2012, 2, 8145–8156. [Google Scholar] [CrossRef]
- Wang, J.H.; Lee, C.S.; Lin, M.C. Mechanism of ethanol reforming: Theoretical foundations. J. Phys. Chem. C. 2009, 113, 6681–6688. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Yoo, S.; Yoo, J.; Park, S.; Gim, M.Y.; Kim, T.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni/Al2O3-La2O3 xerogel catalysts. Mol. Catal. 2017, 434, 123–133. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saxena, N.; Bhatt, A.; Rajagopal, C.; Roy, P.K. Synthesis of mesoporous bimetallic Ni-Cu catalysts supported over ZrO2 by a homogenous urea coprecipitation method for catalytic steam reforming of ethanol. Catal. Sci. Technol. 2013, 3, 1017–1026. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Zhan, E.; Van Veen, A.C.; Mirodatos, C.; Shen, W. Hydrogen production from ethanol over Ir/CeO2 catalysts: A comparative study of steam reforming, partial oxidation and oxidative steam reforming. J. Catal. 2008, 257, 96–107. [Google Scholar] [CrossRef]
- Courty, P.; Ajot, H.; Marcilly, C.; Delmon, B. Oxydesmixtesou en solution solide sous formetrèsdiviséeobtenus par décompositionthermique de précurseursamorphes. Powder Technol. 1973, 7, 21–38. [Google Scholar] [CrossRef]
- Lachowska, M. Steam reforming of methanol over Cu/Zn/Zr/Ga catalyst: Effect of the reduction conditions on the catalytic performance. Reac. Kinet. Mech. Cat. 2010, 101, 85–91. [Google Scholar] [CrossRef]
- Żelazny, A.; Samson, K.; Grabowski, R.; Śliwa, M.; Ruggiero-Mikołajczyk, M.; Kornas, A. Hydrogenolysis of glycerol to propylene glycol over Cu/oxide catalysts: Influence of the support and reaction conditions. Reac. Kinet. Mech. Cat. 2017, 121, 329–343. [Google Scholar] [CrossRef]
- Szarawara, J.; Skrzypek, J.; Gawdzik, A. Podstawy Inżynierii Reaktorów Chemicznych, 2nd ed.; WNT: Warsaw, Poland, 1991; pp. 20–45. [Google Scholar]









| Catalyst | CuO (mass%) | ZrO2 (mass%) | Other Metal Oxides a (mass%) | Size of Crystallites b (nm) | SCu c (m2/gCu) | SBET d (m2/g) | Vp (cm3/g) | Dp (nm) | DCu (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cu0 (111) | ZrO2 (111) | |||||||||
| Cu/Zr/Ni | 63.3 | 32.7 | 4 | 37.8 | 6.3 | 13 | 40 | 0.14 | 13 | 2.0 |
| Cu/Zr/Co | 63.3 | 32.7 | 4 | 32.8 | 5.6 | 11 | 32 | 0.12 | 15 | 1.7 |
| Cu/Zr/Ce | 63.3 | 32.7 | 4 | 75.0 | 4.7 | 6 | 35 | 0.13 | 15 | 0.9 |
| Cu/Zr | 63.8 | 36.2 | – | 23.1 | 5.5 | 3 | 23 | 0.07 | 13 | 0.5 |
| Catalyst | Basic Sites (µmol/g) | Amount of CO2 Adsorbed (µmol) | Amount of CO2 Desorbed (µmol) | Total Basicity (µmol/g) | ||
|---|---|---|---|---|---|---|
| Weak | Medium | Strong | ||||
| CuZrNi | 40.4 | 39.6 | – | 3.9 | 4.0 | 79.9 |
| CuZrCo | 25.1 | 42.8 | – | 3.3 | 3.3 | 67.9 |
| CuZrCe | 34.2 | 45.4 | 16.4 | 5.1 | 4.9 | 96.0 |
| CuZr | 41.3 | 13.9 | 1.0 | 2.9 | 2.8 | 56.2 |
| Catalyst | (L/kgcat·h) | T (K) | α (%) | SCO (%) | (%) |
|---|---|---|---|---|---|
| Cu/Zr/Ni | 490 | 593 | 100 | 5.3 | 35.9 |
| Cu/Zr/Co | 360 | 573 | 97 | 1.8 | 0.3 |
| Cu/Zr/Ce | 378 | 573 | 99 | 0.1 | 0.0 |
| Cu/Zr | 309 | 533 | 91 | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamryszak, Ł.; Kulawska, M.; Madej-Lachowska, M.; Śliwa, M.; Samson, K.; Ruggiero-Mikołajczyk, M. Copper Tricomponent Catalysts Application for Hydrogen Production from Ethanol. Catalysts 2021, 11, 575. https://doi.org/10.3390/catal11050575
Hamryszak Ł, Kulawska M, Madej-Lachowska M, Śliwa M, Samson K, Ruggiero-Mikołajczyk M. Copper Tricomponent Catalysts Application for Hydrogen Production from Ethanol. Catalysts. 2021; 11(5):575. https://doi.org/10.3390/catal11050575
Chicago/Turabian StyleHamryszak, Łukasz, Maria Kulawska, Maria Madej-Lachowska, Michał Śliwa, Katarzyna Samson, and Małgorzata Ruggiero-Mikołajczyk. 2021. "Copper Tricomponent Catalysts Application for Hydrogen Production from Ethanol" Catalysts 11, no. 5: 575. https://doi.org/10.3390/catal11050575