Oxidation of Aqueous Toluene by Gas-Phase Pulsed Corona Discharge in Air-Water Mixtures Followed by Photocatalytic Exhaust Air Cleaning
Abstract
:1. Introduction
2. Results and Discussion
2.1. PCD Oxidation of Aqueous and Gaseous Toluene
2.2. Photocatalytic Treatment of Toluene and Ozone Residues in the PCD Exhaust
3. Materials and Methods
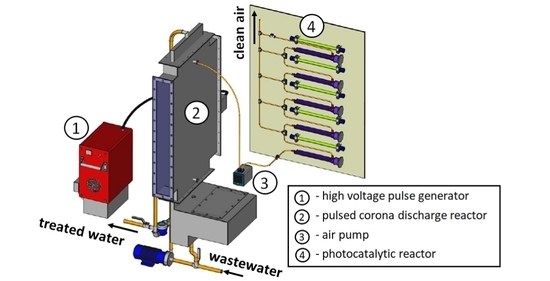
3.1. Experimental Equipment and Procedure
3.1.1. Pulsed Corona Discharge
3.1.2. Photocatalytic Oxidation
3.2. Chemicals and Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalana, M. Investigation of green functionalization of multiwall carbon nanotubes and its application in adsorption of benzene, toluene & p-xylene from aqueous solution. J. Clean. Prod. 2019, 221, 323–338. [Google Scholar] [CrossRef]
- Cseri, L.; Razali, M.; Pogany, P.; Szekely, G. Chapter 3.15—Organic Solvents in Sustainable Synthesis and Engineering. In Green Chemistry: An Inclusive Approach, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 513–553. [Google Scholar] [CrossRef]
- Mrowiec, B. Toluene in sewage and sludge in wastewater treatment plants. Water Sci. Technol. 2014, 69, 128–134. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Air Quality Guidelines for Europe, 2nd ed.; Chapter 5.14—Toluene; WHO Regional Publications: Copenhagen, Denmark, 2000; p. 112. [Google Scholar]
- Leusch, F.; Bartkow, M. A Short Primer on Benzene, Toluene, Ethylbenzene and Xylenes (BTEX) in the Environment and in Hydraulic Fracturing Fluids; Smart Water Research Centre, Griffith University: Queensland, Australia, 2010. [Google Scholar]
- Toluene. Available online: https://www.epa.gov/sites/production/files/2016-09/documents/toluene.pdf (accessed on 30 March 2021).
- Mo, J.; Zhang, Y.; Xu, Q.; Joaquin, J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Debono, O.; Thevenet, F.; Gravejat, P.; Hequet, V.; Raillard, C.; Lecoq, L.; Locoge, N. Toluene photocatalytic oxidation at ppbv levels: Kinetic investigation and carbon balance determination. Appl. Catal. B Environ. 2011, 106, 600–608. [Google Scholar] [CrossRef]
- Bolobajev, J.; Bilgin Öncü, N.; Viisimaa, M.; Trapido, M.; Balcioğlu, I.; Goi, A. Column experiment on activation aids and biosurfactant application to the persulphate treatment of chlorophene-contaminated soil. Environ. Technol. 2015, 36, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kask, M.; Krichevskaya, M.; Bolobajev, J. Sonolytic degradation of pesticide metazachlor in water: The role of dissolved oxygen and ferric sludge in the process intensification. J. Environ. Chem. Eng. 2019, 7, 103095. [Google Scholar] [CrossRef]
- Kask, M.; Bolobajev, J.; Krichevskaya, M. Gas-phase photocatalytic degradation of acetone and toluene, and their mixture in the presence of ozone in a continuous multi-section reactor as possible air post-treatment for exhaust from pulsed corona discharge. Chem. Eng. J. 2020, 399, 125815. [Google Scholar] [CrossRef]
- Jose, J.; Philip, L. Continuous flow pulsed power plasma reactor for the treatment of aqueous solution containing volatile organic compounds and real pharmaceutical wastewater. J. Environ. Manag. 2021, 286, 112202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Zhang, H.; Wu, A.; Yan, J.; Ni, M.; Zhang, H.; Buekens, A. Destruction of toluene by rotating gliding arc discharge. Fuel 2016, 176, 78–85. [Google Scholar] [CrossRef]
- Huang, H.; Ye, D.; Leung, Y.C.; Feng, F.; Guan, X. Byproducts and pathways of toluene destruction via plasma-catalysis. J. Mol. Catal. A Chem. 2011, 336, 87–93. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, N.; Li, J.; Lu, N.; Shang, K.; Wu, Y. An improved corona discharge ignited by oxide cathodes with high secondary electron emission for toluene degradation. Chem. Eng. J. 2019, 362, 339–348. [Google Scholar] [CrossRef]
- Bolobajev, J.; Gornov, D.; Kornev, I.; Preis, S. Degradation of aqueous alachlor in pulsed corona discharge. J. Electrostat. 2021, 109, 103543. [Google Scholar] [CrossRef]
- Kask, M.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of aqueous N-nitrosodiethylamine: Experimental comparison of pulsed corona discharge with H2O2-assisted ozonation. J. Environ. Chem. Eng. 2021, 9, 105102. [Google Scholar] [CrossRef]
- Tikker, P.; Dulova, N.; Kornev, I.; Preis, S. Effects of persulfate and hydrogen peroxide on oxidation of oxalate by pulsed corona discharge. Chem. Eng. J. 2021, 411, 128586. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed corona discharge induced hydroxyl radical transfer through the gas-liquid interface. Sci. Rep. 2017, 7, 16152. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.; Panorel, I.C.; Kornev, I.; Hatakka, H.; Kallas, J. Pulsed corona discharge: The role of ozone and hydroxyl radical in aqueous pollutants oxidation. Water Sci. Technol. 2013, 68, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Rataj, R.; Kolb, J.F.; Bláha, L. Cylindrospermopsin is effectively degraded in water by pulsed corona-like and dielectric barrier discharges. Environ. Pollut. 2020, 266, 115423. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Philip, L. Comparative study of degradation of toluene and methyl isobutyl ketone (MIBK) in aqueous solution by pulsed corona discharge plasma. J. Environ. Sci. 2021, 101, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.; Klauson, D.; Gregor, A. Potential of electric discharge plasma methods in abatement of volatile organic compounds originating from the food industry. J. Environ. Manag. 2013, 114, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Li, J.; Liang, W.; Zhu, T.; Li, Y.; Jin, Y.; He, L. Volatile organic compounds (VOCs) removal by using dielectric barrier discharge. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; IEEE: New York, NY, USA. [Google Scholar] [CrossRef]
- van Durme, J.; Dewulf, J.; Leys, C.; van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B 2008, 78, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Schiorlin, M.; Marotta, E.; Rea, M.; Paradisi, C. Comparison of toluene removal in air at atmospheric conditions by different corona discharges. Environ. Sci. Technol. 2009, 43, 9386–9392. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Minamitani, Y.; Schoenbach, K.H. Comparison of catalytic activity of aluminum oxide and silica gel for decomposition of volatile organic compounds (VOCs) in a plasmacatalytic reactor. IEEE Trans. Plasma Sci. 2005, 33, 50–56. [Google Scholar] [CrossRef]
- Onga, L.; Kornev, I.; Preis, S. Oxidation of reactive azo-dyes with pulsed corona discharge: Surface reaction enhancement. J. Electrostat. 2020, 103, 103420. [Google Scholar] [CrossRef]
- Panorel, I.C.; Preis, S.; Kornev, I.; Hatakka, H.; Louhi-Kultanen, M. Oxidation of aqueous paracetamol by pulsed corona discharge. Ozone Sci. Eng. 2013, 35, 116–124. [Google Scholar] [CrossRef]
- Kornev, I.; Osokin, G.; Galanov, A.; Yavorovskiy, N.; Preis, S. Formation of nitrite- and nitrate-ions in aqueous solutions treated with pulsed electric discharges. Ozone Sci. Eng. 2013, 35, 22–30. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2009; p. 109. [Google Scholar] [CrossRef]
- Pichat, P.; Disdier, J.; Hoang-Van, C.; Mas, D.; Goutailler, G.; Gaysse, C. Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis. Catal. Today 2000, 63, 363–369. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Carbon Monoxide—Leading Cause of Poisoning Deaths. Available online: https://www.creia.org/carbon-monoxide---leading-cause-of-poisoning-deaths (accessed on 30 March 2021).
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Leighton, D.T.; Calo, J.M. Distribution Coefficients of Chlorinated Hydrocarbons in Dilute Air-Water Systems for Groundwater Contamination Applications. J. Chem. Eng. Data 1981, 26, 382–385. [Google Scholar] [CrossRef]
- Kornev, I.; Saprykin, F.; Preis, S. Stability and energy efficiency of pulsed corona discharge in treatment of dispersed high-conductivity aqueous solutions. J. Electrostat. 2017, 89, 42–50. [Google Scholar] [CrossRef]






| PCD Treatment Conditions | k2 × 10−7, m3·J−1 | R2 | Efficiency of Toluene Oxidation for 40%, g·kW−1·h−1 | ||
|---|---|---|---|---|---|
| Aqueous | Airborne | ||||
| (a) | C0 = 6.3 mg·L−1, 880 pps, initial pH 7.0, 20 °C | 6.9 | 0.9981 | 7.5 | 22.3 |
| (b) | C0 = 6.3 mg·L−1, 200 pps, initial pH 7.0, 20 °C | 8.3 | 0.9964 | 8.6 | 27.0 |
| (c) | C0 = 6.3 mg·L−1, 50 pps, initial pH 7.0, 20 °C | 10.7 | 0.9905 | 10.5 | 29.3 |
| (d) | C0 = 3.7 mg·L−1, 200 pps, initial pH 7.0, 20 °C | 12.4 | 0.9994 | 7.5 | 23.0 |
| (e) | C0 = 1.0 mg·L−1, 200 pps, initial pH 7.0, 20 °C | 37.0 | 0.9938 | 6.4 | 13.7 |
| (f) | C0 = 3.7 mg·L−1, 200 pps, pH 3.0, 20 °C | 12.5 | 0.9887 | 7.6 | 22.4 |
| (g) | C0 = 3.7 mg·L−1, 200 pps, pH 12.0, 20 °C | 13.0 | 0.9943 | 7.6 | 24.9 |
| (h) | C0 = 2.5 mg·L−1, 200 pps, initial pH 7.0, 30 °C | 12.2 | 0.9984 | 5.3 | 29.5 |
| PCD Treatment Conditions | Sampling Time, min | Concentration, mg·L−1 | |||
|---|---|---|---|---|---|
| Toluene | O3 | CO | |||
| (a) | C0 = 6.3 mg·L−1 (aq), C0 = 1.6 mg·L−1 (gas), 880 pps, initial pH 7.0, 20 °C | 6 | 0.057 | 0.61 | 0.051 |
| (b) | C0 = 6.3 mg·L−1 (aq), C0 = 1.6 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 20 | 0.080 | 0.40 | 0.042 |
| (c) | C0 = 6.3 mg·L−1 (aq), C0 = 1.6 mg·L−1 (gas), 50 pps, initial pH 7.0, 20 °C | 60 | 0.10 | 0.22 | 0.033 |
| (d) | C0 = 3.7 mg·L−1 (aq), C0 = 1.0 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 12.5 | 0.061 | 0.39 | 0.028 |
| (e) | C0 = 1.0 mg·L−1 (aq), C0 = 0.4 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 5 | 0.065 | 0.34 | 0.016 |
| (f) | C0 = 3.7 mg·L−1 (aq), C0 = 1.0 mg·L−1 (gas), 200 pps, pH 3.0, 20 °C | 12.5 | 0.057 | 0.42 | 0.030 |
| (g) | C0 = 3.7 mg·L−1 (aq), C0 = 1.0 mg·L−1 (gas), 200 pps, pH 12.0, 20 °C | 12.5 | 0.065 | 0.044 | 0.030 |
| (h) | C0 = 2.5 mg·L−1 (aq), C0 = 1.2 mg·L−1 (gas), 200 pps, initial pH 7.0, 30 °C | 12.5 | 0.096 | 0.22 | 0.029 |
| PCD Treatment Conditions | Sampling Time, min | Concentration, mg·L−1 | ||
|---|---|---|---|---|
| Toluene | O3 | CO | ||
| C0 = 6.3 mg·L−1 (aq), C0 = 1.6 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 20 | 0.034 | 0.29 | 0.038 |
| C0 = 3.7 mg·L−1 (aq), C0 = 1.0 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 12.5 | 0.046 | 0.29 | 0.024 |
| C0 = 1.0 mg·L−1 (aq), C0 = 0.4 mg·L−1 (gas), 200 pps, initial pH 7.0, 20 °C | 5 | 0.042 | 0.24 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kask, M.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of Aqueous Toluene by Gas-Phase Pulsed Corona Discharge in Air-Water Mixtures Followed by Photocatalytic Exhaust Air Cleaning. Catalysts 2021, 11, 549. https://doi.org/10.3390/catal11050549
Kask M, Krichevskaya M, Preis S, Bolobajev J. Oxidation of Aqueous Toluene by Gas-Phase Pulsed Corona Discharge in Air-Water Mixtures Followed by Photocatalytic Exhaust Air Cleaning. Catalysts. 2021; 11(5):549. https://doi.org/10.3390/catal11050549
Chicago/Turabian StyleKask, Maarja, Marina Krichevskaya, Sergei Preis, and Juri Bolobajev. 2021. "Oxidation of Aqueous Toluene by Gas-Phase Pulsed Corona Discharge in Air-Water Mixtures Followed by Photocatalytic Exhaust Air Cleaning" Catalysts 11, no. 5: 549. https://doi.org/10.3390/catal11050549