Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation
Abstract
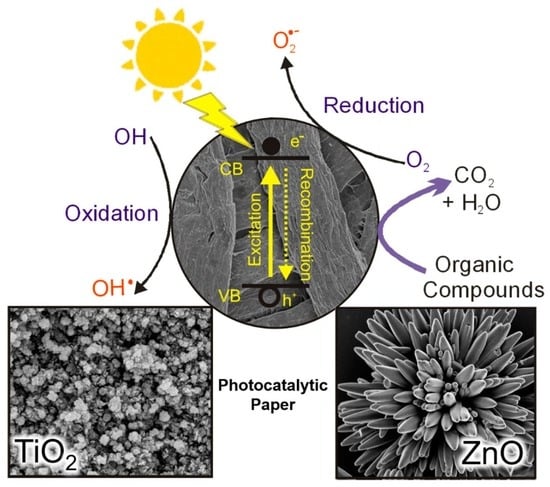
:1. Introduction
1.1. Cellulose-Based Materials
1.2. Photocatalytic Paper

1.3. Metal Oxide Nanostructures and Thin Films
2. Metal Oxide Photocatalytic Papers
2.1. TiO2 Photocatalytic Paper
2.2. ZnO Photocatalytic Paper

3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vicente, A.; Araujo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Aguas, H.; Fortunato, E.; Martins, R. Multifunctional cellulose-paper for light harvesting and smart sensing applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd Mutalib, M.; Hir, Z.A.M.; Zain, M.F.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N.W. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Festucci-Buselli, R.A.; Otoni, W.C.; Joshi, C.P. Structure, organization, and functions of cellulose synthase complexes in higher plants. Braz. J. Plant Physiol. 2007, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose; Intech: Rijeka, Croatia, 2013; Volume 2, pp. 45–68. [Google Scholar]
- Gaspar, D.; Fernandes, S.N.; de Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnology 2014, 25, 094008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, E.; Gaspar, D.; Duarte, P.; Pereira, L.; Águas, H.; Vicente, A.; Dourado, F.; Gama, M.; Martins, R. Chapter 11—Optoelectronic Devices from Bacterial NanoCellulose. In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 179–197. [Google Scholar] [CrossRef]
- Gama, M.; Dourado, F.; Bielecki, S. Bacterial Nanocellulose: From Biotechnology to Bio-Economy; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zschieschang, U.; Klauk, H. Organic transistors on paper: A brief review. J. Mater. Chem. C 2019, 7, 5522–5533. [Google Scholar] [CrossRef] [Green Version]
- Vicente, A.; Águas, H.; Mateus, T.; Araújo, A.; Lyubchyk, A.; Siitonen, S.; Fortunato, E.; Martins, R. Solar cells for self-sustainable intelligent packaging. J. Mater. Chem. A 2015, 3, 13226–13236. [Google Scholar] [CrossRef]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef] [Green Version]
- Matias, M.L.; Nunes, D.; Pimentel, A.; Ferreira, S.H.; Borda d Agua, R.; Duarte, M.P.; Fortunato, E.; Martins, R. Paper-Based Nanoplatforms for Multifunctional Applications. J. Nanomater. 2019, 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, D.; Freire, T.; Barranger, A.; Vieira, J.; Matias, M.; Pereira, S.; Pimentel, A.; Cordeiro, N.J.; Fortunato, E.; Martins, R. TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Appl. Sci. 2020, 10, 1200. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.W.; Österholm, A.M.; Reynolds, J.R. Paper-Based Electrochromic Devices Enabled by Nanocellulose-Coated Substrates. Adv. Funct. Mater. 2019, 29, 1903487. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 094006. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Araujo, A.; Calmeiro, T.; Panigrahi, S.; Pinto, J.; Barquinha, P.; Gama, M.; Fortunato, E.; Martins, R. Enhanced UV Flexible Photodetectors and Photocatalysts Based on TiO2 Nanoplatforms. Top. Catal. 2018, 61, 1591–1606. [Google Scholar] [CrossRef] [Green Version]
- Baruah, S.; Jaisai, M.; Imani, R.; Nazhad, M.M.; Dutta, J. Photocatalytic paper using zinc oxide nanorods. Sci. Technol. Adv. Mater. 2010, 11, 055002. [Google Scholar] [CrossRef]
- Yan, L.; Liu, B.; Li, W.; Zhao, T.; Wang, Y.; Zhao, Q. Multiscale cellulose based self-assembly of hierarchical structure for photocatalytic degradation of organic pollutant. Cellulose 2020, 27, 5241–5253. [Google Scholar] [CrossRef]
- Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 20 February 2021).
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Marschall, R.; Wang, L. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Chapter 6—Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef] [Green Version]
- Khokhra, R.; Bharti, B.; Lee, H.-N.; Kumar, R. Visible and UV photo-detection in ZnO nanostructured thin films via simple tuning of solution method. Sci. Rep. 2017, 7, 15032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Yáñez, E.; Santander, P.; Contreras, D.; Yáñez, J.; Cornejo, L.; Mansilla, H.D. Homogeneous and heterogeneous degradation of caffeic acid using photocatalysis driven by UVA and solar light. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 78–85. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic Activity of TiO2 Nanostructured Arrays Prepared by Microwave-Assisted Solvothermal Method. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; Cao, W., Ed.; InTech: Rijeka, Croatia, 2016; Chapter 3. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278 Pt 2, 262–270. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B Environ. 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Beydoun, D.R.; Amal, R.; Low, G.; McEvoy, S. Role of Nanoparticles in Photocatalysis. J. Nanoparticle Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Hong, X.; Wang, Z.; Cai, W.; Lu, F.; Zhang, J.; Yang, Y.; Ma, N.; Liu, Y. Visible-Light-Activated Nanoparticle Photocatalyst of Iodine-Doped Titanium Dioxide. Chem. Mater. 2005, 17, 1548–1552. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.; Yin, K.; Dong, S.; Ren, Z.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.M. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Wang, R.; Cai, X.; Shen, F. Preparation of TiO2 hollow microspheres by a novel vesicle template method and their enhanced photocatalytic properties. Ceram. Int. 2013, 39, 9465–9470. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Mader, E.; Ma, P.-C. Controlled synthesis of hierarchical TiO2 nanoparticles on glass fibres and their photocatalytic performance. Dalton Trans. 2014, 43, 12743–12753. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Amemiya, T.; Murabayashi, M.; Itoh, K. Photocatalytic Activity of Sol−Gel TiO2 Thin Films on Various Kinds of Glass Substrates: The Effects of Na+ and Primary Particle Size. J. Phys. Chem. B 2004, 108, 8254–8259. [Google Scholar] [CrossRef]
- Rampaul, A.; Parkin, I.P.; O’Neill, S.A.; DeSouza, J.; Mills, A.; Elliott, N. Titania and tungsten doped titania thin films on glass; active photocatalysts. Polyhedron 2003, 22, 35–44. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Enhanced Rates of Photocatalytic Degradation of an Azo Dye Using SnO2/TiO2 Coupled Semiconductor Thin Films. Environ. Sci. Technol. 1995, 29, 841–845. [Google Scholar] [CrossRef]
- Qi, K.; Xin, J.H. Room-Temperature Synthesis of Single-Phase Anatase TiO2 by Aging and its Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2010, 2, 3479–3485. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Yeh, S.-M.; Chen, C.-H.; Lin, H.-N. Flexible Photocatalytic Paper with Cu2O and Ag Nanoparticle-Decorated ZnO Nanorods for Visible Light Photodegradation of Organic Dye. Nanoscale Res. Lett. 2019, 14, 204. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, K.; Kuwahara, Y.; Sumida, Y.; Yamashita, H. Fabrication of Photocatalytic Paper Using TiO2 Nanoparticles Confined in Hollow Silica Capsules. Langmuir 2017, 33, 288–295. [Google Scholar] [CrossRef]
- Pelton, R.; Geng, X.; Brook, M. Photocatalytic paper from colloidal TiO2--fact or fantasy. Adv. Colloid Interface Sci. 2006, 127, 43–53. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Wang, P.; Qian, K. Photocatalytic behavior of cellulose-based paper with TiO2 loaded on carbon fibers. J. Environ. Chem. Eng. 2013, 1, 175–182. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, W.; Chen, H.; Chen, J.; Wang, H.; Song, Z. Preparing photocatalytic paper with improved catalytic activity by in situ loading poly-dopamine on cellulose fibre. Bull. Mater. Sci. 2019, 42, 54. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Mater. Chem. C 2014, 2, 6097–6103. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Wen Lou, X. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef] [Green Version]
- Devan, R.S.; Patil, R.A.; Lin, J.-H.; Ma, Y.-R. One-Dimensional Metal-Oxide Nanostructures: Recent Developments in Synthesis, Characterization, and Applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Ansari, A.A.; Alhoshan, M.; Alsalhi, M.; Aldwayyan, A. Nanostructured metal oxides based enzymatic electrochemical biosensors. In Biosensors; InTech: Rijeka, Croatia, 2010. [Google Scholar] [CrossRef] [Green Version]
- Walia, S.; Balendhran, S.; Nili, H.; Zhuiykov, S.; Rosengarten, G.; Wang, Q.H.; Bhaskaran, M.; Sriram, S.; Strano, M.S.; Kalantar-zadeh, K. Transition metal oxides—Thermoelectric properties. Prog. Mater. Sci. 2013, 58, 1443–1489. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S. Fe2O3-photocatalysis with sunlight and UV light: Oxidation of aniline. Electrochem. Commun. 2006, 8, 95–101. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified titanium dioxide for photocatalytic applications. In Photocatalysts—Applications and Attributes; Intech: London, UK, 2019; Volume 18. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Santos, L.; Pimentel, A.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. Metal Oxide Nanostructures: Synthesis, Properties and Applications; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Chaurasia, A.K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: Degradation of alizarin red S dye. J. Environ. Chem. Eng. 2017, 5, 2730–2739. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Deuermeier, J.; Freire, T.; Fortunato, E.; Martins, R.; Barquinha, P. Shape Effect of Zinc-Tin Oxide Nanostructures on Photodegradation of Methylene Blue and Rhodamine B under UV and Visible Light. ACS Appl. Nano Mater. 2021, 4, 1149–1161. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Dittmann, R. 9—Stoichiometry in epitaxial oxide thin films. In Epitaxial Growth of Complex Metal Oxides; Koster, G., Huijben, M., Rijnders, G., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 231–261. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.-B.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, G.; Hu, Y.; Fan, G.; Tsang, Y.H.; Li, Z.; Zou, Z. Two-dimensional nanomaterials for photocatalytic CO2 reduction to solar fuels. Sustain. Energy Fuels 2017, 1, 1875–1898. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Sugunan, A.; Warad, H.C.; Boman, M.; Dutta, J. Zinc oxide nanowires in chemical bath on seeded substrates: Role of hexamine. J. Sol-Gel Sci. Technol. 2006, 39, 49–56. [Google Scholar] [CrossRef]
- Han, S.-Y.; Lee, D.-H.; Chang, Y.-J.; Ryu, S.-O.; Lee, T.-J.; Chang, C.-H. The growth mechanism of nickel oxide thin films by room-temperature chemical bath deposition. J. Electrochem. Soc. 2006, 153, C382. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angew. Chem. Int. Ed. Engl. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Kim, I.D.; Rothschild, A. Nanostructured metal oxide gas sensors prepared by electrospinning. Polym. Adv. Technol. 2011, 22, 318–325. [Google Scholar] [CrossRef]
- Monk, P.; Chester, S.L. Electro-deposition of films of electrochromic tungsten oxide containing additional metal oxides. Electrochim. Acta 1993, 38, 1521–1526. [Google Scholar] [CrossRef]
- Bohannan, E.W.; Shumsky, M.G.; Switzer, J.A. Epitaxial electrodeposition of copper (I) oxide on single-crystal gold (100). Chem. Mater. 1999, 11, 2289–2291. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal oxide thin films prepared by magnetron sputtering technology for volatile organic compound detection in the microwave frequency range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, E.; Barros, R.; Barquinha, P.; Figueiredo, V.; Park, S.-H.K.; Hwang, C.-S.; Martins, R. Transparent p-type SnOx thin film transistors produced by reactive rf magnetron sputtering followed by low temperature annealing. J. Appl. Phys. Lett. 2010, 97, 052105. [Google Scholar] [CrossRef]
- Assuncao, V.; Fortunato, E.; Marques, A.; Aguas, H.; Ferreira, I.; Costa, M.; Martins, R.J.T.S.F. Influence of the deposition pressure on the properties of transparent and conductive ZnO: Ga thin-film produced by rf sputtering at room temperature. Thin Solid Film. 2003, 427, 401–405. [Google Scholar] [CrossRef]
- Rodrigues, J.; Fernandes, A.J.; Monteiro, T.; Costa, F.M.J.C. A review on the laser-assisted flow deposition method: Growth of ZnO micro and nanostructures. CrystEngComm 2019, 21, 1071–1090. [Google Scholar] [CrossRef]
- Santos, N.; Rodrigues, J.; Holz, T.; Sedrine, N.B.; Sena, A.; Neves, A.; Costa, F.; Monteiro, T. Luminescence studies on SnO2 and SnO2: Eu nanocrystals grown by laser assisted flow deposition. Phys. Chem. Chem. Phys. 2015, 17, 13512–13519. [Google Scholar] [CrossRef]
- Pavan, M.; Rühle, S.; Ginsburg, A.; Keller, D.A.; Barad, H.-N.; Sberna, P.M.; Nunes, D.; Martins, R.; Anderson, A.Y.; Zaban, A. TiO2/Cu2O all-oxide heterojunction solar cells produced by spray pyrolysis. Sol. Energy Mater. Sol. Cells 2015, 132, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Sutorik, A.C.; Laine, R.M.; Marchal, J.; Johns, T.; Hinklin, T. Mixed-Metal Oxide Particles by Liquid Feed Flame Spray Pyrolysis of Oxide Precursors in Oxygenated Solvents. U.S. Patent 7,220,398, 22 May 2007. [Google Scholar]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Araujo, A.; Coelho, B.J.; Nunes, D.; Oliveira, M.J.; Mendes, M.J.; Aguas, H.; Martins, R.; Fortunato, E. 3D ZnO/Ag Surface-Enhanced Raman Scattering on Disposable and Flexible Cardboard Platforms. Materials 2017, 10, 1351. [Google Scholar] [CrossRef] [Green Version]
- Chirayil, T.; Zavalij, P.Y.; Whittingham, M.S. Hydrothermal synthesis of vanadium oxides. Chem. Mater. 1998, 10, 2629–2640. [Google Scholar] [CrossRef]
- Kardarian, K.; Nunes, D.; Sberna, P.M.; Ginsburg, A.; Keller, D.A.; Pinto, J.V.; Deuermeier, J.; Anderson, A.Y.; Zaban, A.; Martins, R. Effect of Mg doping on Cu2O thin films and their behavior on the TiO2/Cu2O heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 27–36. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef] [Green Version]
- Ha, L.P.P.; Vinh, T.H.T.; Thuy, N.T.B.; Thi, C.M.; Viet, P.V. Visible-light-driven photocatalysis of anisotropic silver nanoparticles decorated on ZnO nanorods: Synthesis and characterizations. J. Environ. Chem. Eng. 2021, 9, 105103. [Google Scholar] [CrossRef]
- Di Mauro, A.; Zimbone, M.; Fragalà, M.E.; Impellizzeri, G. Synthesis of ZnO nanofibers by the electrospinning process. Mater. Sci. Semicond. Process. 2016, 42, 98–101. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.; Jana, S.C.J.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Nalbandian, M.J.; Greenstein, K.E.; Shuai, D.; Zhang, M.; Choa, Y.-H.; Parkin, G.F.; Myung, N.V.; Cwiertny, D.M. Tailored Synthesis of Photoactive TiO2 Nanofibers and Au/TiO2 Nanofiber Composites: Structure and Reactivity Optimization for Water Treatment Applications. Environ. Sci. Technol. 2015, 49, 1654–1663. [Google Scholar] [CrossRef]
- Pei, C.C.; Leung, W.W.-F. Photocatalytic degradation of Rhodamine B by TiO2/ZnO nanofibers under visible-light irradiation. Sep. Purif. Technol. 2013, 114, 108–116. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Ren, H.; Guo, F.; Huang, X.; Shi, Y.; Tang, Y. Construction of a 0D/1D composite based on Au nanoparticles/CuBi2O4 microrods for efficient visible-light-driven photocatalytic activity. Beilstein J. Nanotechnol. 2019, 10, 1360–1367. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.; Chen, X.; Chen, C.; Chen, P.; Wang, Z.; Duan, Y. Functional Metal Oxides in Perovskite Solar Cells. ChemPhysChem 2019, 20, 2580–2586. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Aguas, H.; Fortunato, E.; Martins, R. Inkjet printed and “doctor blade” TiO2 photodetectors for DNA biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Raible, I.; Burghard, M.; Schlecht, U.; Yasuda, A.; Vossmeyer, T. V2O5 nanofibres: Novel gas sensors with extremely high sensitivity and selectivity to amines. Sens. Actuators B Chem. 2005, 106, 730–735. [Google Scholar]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, S. Electrospun Metal Oxide Composite Nanofibers Gas Sensors: A Review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Y. Recent Progress of TiO2-Based Anodes for Li Ion Batteries. J. Nanomater. 2016, 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cao, X.; Bourgeois, L.; Guan, H.; Chen, S.; Zhong, Y.; Tang, D.M.; Li, H.; Zhai, T.; Li, L. N-Doped Graphene-SnO2 Sandwich Paper for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2012, 22, 2682–2690. [Google Scholar] [CrossRef]
- Subalakshmi, P.; Sivashanmugam, A. CuO nano hexagons, an efficient energy storage material for Li-ion battery application. J. Alloys Compd. 2017, 690, 523–531. [Google Scholar] [CrossRef]
- Lee, J.; Jo, C.; Park, B.; Hwang, W.; Lee, H.I.; Yoon, S.; Lee, J.J.N. Simple fabrication of flexible electrodes with high metal-oxide content: Electrospun reduced tungsten oxide/carbon nanofibers for lithium ion battery applications. Nanoscale 2014, 6, 10147–10155. [Google Scholar] [CrossRef] [Green Version]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Rezaei Darvishi, S.M. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Rathee, D.; Arya, S.; Kumar, M. Analysis of TiO2 for microelectronic applications: Effect of deposition methods on their electrical properties. Front. Optoelectron. China 2011, 4, 349–358. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Rocquefelte, X.; Goubin, F.; Koo, H.-J.; Whangbo, M.-H.; Jobic, S. Investigation of the Origin of the Empirical Relationship between Refractive Index and Density on the Basis of First Principles Calculations for the Refractive Indices of Various TiO2 Phases. Inorg. Chem. 2004, 43, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-D.; Ching, W.Y. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B 1995, 51, 13023–13032. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Structural Characteristics and Mechanical and Thermodynamic Properties of Nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613–9644. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A Patterned TiO2(Anatase)/TiO2(Rutile) Bilayer-Type Photocatalyst: Effect of the Anatase/Rutile Junction on the Photocatalytic Activity. Angew. Chem. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable Method for the Synthesis of Biphasic TiO2 Nanocrystals with Tunable Phase Composition and Enabled Visible-Light Photocatalytic Performance. ACS Appl. Mater. Interfaces 2012, 4, 1239–1246. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Nakajima, H.; Mori, T.; Shen, Q.; Toyoda, T. Photoluminescence study of mixtures of anatase and rutile TiO2 nanoparticles: Influence of charge transfer between the nanoparticles on their photoluminescence excitation bands. Chem. Phys. Lett. 2005, 409, 81–84. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, B.; Huang, L.; Liu, P.; Wang, X.; Lu, Z.; Xu, G.; Zhang, E.; Wang, H.; Kong, Z.; et al. Anatase nano-TiO2 with exposed curved surface for high photocatalytic activity. J. Alloys Compd. 2016, 661, 441–447. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.; Zink, J.I.; Nel, A.E.; Mädler, L. Role of Fe Doping in Tuning the Band Gap of TiO2 for the Photo-Oxidation-Induced Cytotoxicity Paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef] [Green Version]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and Surface Modification of TiO2-Based Photocatalysts for the Conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Wu, M.; Szeto, W.; Wang, Y.; Pan, W.; Leung, D.Y.C. Carbon doped ultra-small TiO2 coated on carbon cloth for efficient photocatalytic toluene degradation under visible LED light irradiation. Appl. Surf. Sci. 2020, 527, 146780. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Al-Hamdani, A.A.S.; Sunghun, B.; Ko, Y.G. A review on TiO2-based composites for superior photocatalytic activity. Rev. Inorg. Chem. 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, G.; Hong, J. Synthesis and characterization of rutile TiO2 nanowhiskers. J. Mater. Res. 1999, 14, 3346–3354. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Yang, H.G.; Zeng, H.C. Preparation of Hollow Anatase TiO2 Nanospheres via Ostwald Ripening. J. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef]
- Lin, J.; Heo, Y.-U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6% conversion efficiency. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kranthi Kiran, A.S.; Madhumathi, K.; Sampath Kumar, T.S. Electrosprayed titania nanocups for protein delivery. Colloid Interface Sci. Commun. 2016, 12, 17–20. [Google Scholar] [CrossRef]
- Negishi, N.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Preparation of transparent TiO2 thin film photocatalyst and its photocatalytic activity. J. Chem. Lett. 1995, 24, 841–842. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Zhao, Q. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Film. 2000, 379, 7–14. [Google Scholar] [CrossRef]
- Evtushenko, Y.M.; Romashkin, S.V.; Trofimov, N.S.; Chekhlova, T.K. Optical Properties of TiO2 Thin Films. Phys. Procedia 2015, 73, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Sagadevan, S. Synthesis and electrical properties of TiO2 nanoparticles using a wet chemical technique. Am. J. Nanosci. Nanotechnol. 2014, 1, 27. [Google Scholar] [CrossRef] [Green Version]
- Fernández, I.E.; Rodríguez-Páez, J.E. Wet-chemical preparation of TiO2-nanostructures using different solvents: Effect of CTAB concentration and tentative mechanism of particle formation. J. Alloys Compd. 2019, 780, 756–771. [Google Scholar] [CrossRef]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T.; Tseng, Y.-K.; Chen, I.C. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J. Cryst. Growth 2005, 281, 384–390. [Google Scholar] [CrossRef]
- Boyadzhiev, S.; Georgieva, V.; Rassovska, M. Characterization of reactive sputtered TiO2 thin films for gas sensor applications. J. Phys. Conf. Ser. 2010, 253, 012040. [Google Scholar] [CrossRef]
- Sanchez-Sobrado, O.; Mendes, M.J.; Haque, S.; Mateus, T.; Araujo, A.; Aguas, H.; Fortunato, E.; Martins, R. Colloidal-lithographed TiO2 photonic nanostructures for solar cell light trapping. J. Mater. Chem. C 2017, 5, 6852–6861. [Google Scholar] [CrossRef]
- Sreekantan, S.; Saharudin, K.A.; Wei, L.C. Formation of TiO2 nanotubes via anodization and potential applications for photocatalysts, biomedical materials, and photoelectrochemical cell. IOP Conf. Ser. Mater. Sci. Eng. 2011, 21, 012002. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A new benchmark for TiO2 nanotube array growth by anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Jiang, L.C.; Zhang, W.D. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 2009, 21, 988–993. [Google Scholar] [CrossRef]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P.J.A.M. Filling of TiO2 Nanotubes by Self-Doping and Electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Kim, S.S.J.A.S. Synthesis of aligned TiO2 nanofibers using electrospinning. Appl. Sci. 2018, 8, 309. [Google Scholar]
- Lee, D.; Rho, Y.; Allen, F.I.; Minor, A.M.; Ko, S.H.; Grigoropoulos, C.P. Synthesis of hierarchical TiO2 nanowires with densely-packed and omnidirectional branches. Nanoscale 2013, 5, 11147–11152. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Xiao, W. Enhanced photoelectrocatalytic performance of SnO2/TiO2 rutile composite films. J. Mater. Chem. A 2013, 1, 10727–10735. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Zhu, Y. Fabrication of porous TiO2 film via hydrothermal method and its photocatalytic performances. Thin Solid Film. 2007, 515, 7127–7134. [Google Scholar] [CrossRef]
- Chen, Q.; Qian, Y.; Chen, Z.; Wu, W.; Chen, Z.; Zhou, G.; Zhang, Y. Hydrothermal epitaxy of highly oriented TiO2 thin films on silicon. Appl. Phys. Lett. 1995, 66, 1608–1610. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B Environ. 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Kondo, T.; Nagao, S.; Yanagishita, T.; Nguyen, N.T.; Lee, K.; Schmuki, P.; Masuda, H. Ideally ordered porous TiO2 prepared by anodization of pretextured Ti by nanoimprinting process. Electrochem. Commun. 2015, 50, 73–76. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, G.H.; Jin, Y.X.; Zhang, Y.; Zhang, J.; Zhang, L.D. Hydrothermal synthesis and photoluminescence of TiO2 nanowires. Chem. Phys. Lett. 2002, 365, 300–304. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Li, G.; An, T.; Yamashita, H. Fabrication of Au/TiO2 nanowires@carbon fiber paper ternary composite for visible-light photocatalytic degradation of gaseous styrene. Catal. Today 2017, 281, 621–629. [Google Scholar] [CrossRef]
- Sboui, M.; Bouattour, S.; Liotta, L.F.; Parola, V.L.; Gruttadauria, M.; Marcì, G.; Boufi, S. Paper-TiO2 composite: An effective photocatalyst for 2-propanol degradation in gas phase. J. Photochem. Photobiol. A Chem. 2018, 350, 142–151. [Google Scholar] [CrossRef]
- Hu, T.; Sun, X.; Sun, H.; Yu, M.; Lu, F.; Liu, C.; Lian, J. Flexible free-standing graphene–TiO2 hybrid paper for use as lithium ion battery anode materials. Carbon 2013, 51, 322–326. [Google Scholar] [CrossRef]
- Hirokazu, M.; Makoto, T.; Shinichi, K.; Kazuhito, H.; Akira, F. Photoactive TiO2 Containing Paper: Preparation and Its Photocatalytic Activity under Weak UV Light Illumination. Chem. Lett. 1995, 24, 767–768. [Google Scholar] [CrossRef]
- Iguchi, Y.; Ichiura, H.; Kitaoka, T.; Tanaka, H. Preparation and characteristics of high performance paper containing titanium dioxide photocatalyst supported on inorganic fiber matrix. Chemosphere 2003, 53, 1193–1199. [Google Scholar] [CrossRef]
- Izadyar, S.; Fatemi, S. Fabrication of X Zeolite Based Modified Nano TiO2 Photocatalytic Paper for Removal of VOC Pollutants under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 10961–10968. [Google Scholar] [CrossRef]
- Sboui, M.; Bouattour, S.; Gruttadauria, M.; Marcì, G.; Liotta, L.F.; Boufi, S. Paper Functionalized with Nanostructured TiO2/AgBr: Photocatalytic Degradation of 2–Propanol under Solar Light Irradiation and Antibacterial Activity. Nanomaterials 2020, 10, 470. [Google Scholar] [CrossRef] [Green Version]
- Barka, N.; Qourzal, S.; Assabbane, A.; Nounah, A.; Ait-Ichou, Y. Factors influencing the photocatalytic degradation of Rhodamine B by TiO2-coated non-woven paper. J. Photochem. Photobiol. A Chem. 2008, 195, 346–351. [Google Scholar] [CrossRef]
- Rehim, M.H.A.; El-Samahy, M.A.; Badawy, A.A.; Mohram, M.E. Photocatalytic activity and antimicrobial properties of paper sheets modified with TiO2/Sodium alginate nanocomposites. Carbohydr. Polym. 2016, 148, 194–199. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Li, J. Anatase TiO2/cellulose hybrid paper: Synthesis, characterizations, and photocatalytic activity for degradation of indigo carmine dye. Funct. Mater. Lett. 2017, 10, 1750018. [Google Scholar] [CrossRef]
- Toro, R.; Diab, M.; De caro, T.; Kamar, M.; Adel, A.; Caschera, D. Study of the Effect of Titanium Dioxide Hydrosol on the Photocatalytic and Mechanical Properties of Paper Sheets. Materials 2020, 13, 1326. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.; Fleming, P.D.; Joyce, M.; Ari-Gur, P. High performance nano-titania photocatalytic paper composite. Part II: Preparation and characterization of natural zeolite-based nano-titania composite sheets and study of their photocatalytic activity. Mater. Sci. Eng. B 2009, 164, 135–139. [Google Scholar] [CrossRef]
- Ko, S.; Pekarovic, J.; Fleming, P.D.; Ari-Gur, P. High performance nano-titania photocatalytic paper composite. Part I: Experimental design study for TiO2 composite sheet using a natural zeolite microparticle system and its photocatalytic property. Mater. Sci. Eng. B 2010, 166, 127–131. [Google Scholar] [CrossRef]
- Adjimi, S.; Sergent, N.; Roux, J.C.; Delpech, F.; Pera-Titus, M.; Chhor, K.; Kanaev, A.; Thivel, P.X. Photocatalytic paper based on sol–gel titania nanoparticles immobilized on porous silica for VOC abatement. Appl. Catal. B Environ. 2014, 154–155, 123–133. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Shen, Y.; Han, J.; Yuan, L.; Gong, L.; Xu, Z.; Bai, X.; Wei, M.; Tong, Y.; et al. Three-dimensional WO3 nanostructures on carbon paper: Photoelectrochemical property and visible light driven photocatalysis. Chem. Commun. 2011, 47, 5804–5806. [Google Scholar] [CrossRef] [PubMed]
- Freire, T.; Fragoso, A.R.; Matias, M.; Pinto, J.V.; Marques, A.C.; Pimentel, A.; Barquinha, P.; Huertas, R.; Fortunato, E.; Martins, R.; et al. Enhanced solar photocatalysis of TiO2 nanoparticles and nanostructured thin films grown on paper. Nano Express 2021. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- Coleman, V.A.; Jagadish, C. Chapter 1—Basic Properties and Applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures; Jagadish, C., Pearton, S., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 1–20. [Google Scholar] [CrossRef]
- Mohammad, V.; Umar, A.; Hahn, Y.-B. ZnO Nanoparticles: Growth, Properties, and Applications. In Metal Oxide Nanostructures and Their Applications; Umar, A., Hahn, Y., Eds.; American Scientific Publishers: Valencia, CA, USA, 2010; Volume 5, pp. 1–36. [Google Scholar]
- Geurts, J. Crystal structure, chemical binding, and lattice properties. In Zinc Oxide; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–37. [Google Scholar]
- Wen, B.; Huang, Y.; Boland, J.J. Controllable Growth of ZnO Nanostructures by a Simple Solvothermal Process. J. Phys. Chem. C 2008, 112, 106–111. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Alshammari, A.S.; Jayawardena, K.D.G.I.; Beliatis, M.J.; Henley, S.J.; Silva, S.R.P. Role of the Exposed Polar Facets in the Performance of Thermally and UV Activated ZnO Nanostructured Gas Sensors. J. Phys. Chem. C 2013, 117, 17850–17858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Liu, Y.; Dong, L.; Zhao, D.; Zhang, J.; Lu, Y.; Shen, D.; Fan, X. Growth of ZnO Nanostructures with Different Morphologies by Using Hydrothermal Technique. J. Phys. Chem. B 2006, 110, 20263–20267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Zhang, Q.; Li, T. Adjusting the proportions of {0001} facets and high-index facets of ZnO hexagonal prisms and their photocatalytic activity. RSC Adv. 2017, 7, 3515–3520. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, J.; Batzill, M. Surface Functionalization of ZnO Photocatalysts with Monolayer ZnS. J. Phys. Chem. C 2008, 112, 4304–4307. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of 0D/3D CuO/ZnO Heterojunction with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]
- Meskine, H.; Mulheran, P.A. Simulation of reconstructions of the polar ZnO$(0001)$ surfaces. Phys. Rev. B 2011, 84, 165430. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Zhang, Z.; Yan, J.; Zhao, W.; Zhai, C.; Liu, J. Growth mechanism and photoluminescence property of hydrothermal oriented ZnO nanostructures evolving from nanorods to nanoplates. J. Alloys Compd. 2017, 718, 161–169. [Google Scholar] [CrossRef]
- Manjula, Y.; Rakesh Kumar, R.; Swarup Raju, P.M.; Anil Kumar, G.; Venkatappa Rao, T.; Akshaykranth, A.; Supraja, P. Piezoelectric flexible nanogenerator based on ZnO nanosheet networks for mechanical energy harvesting. Chem. Phys. 2020, 533, 110699. [Google Scholar] [CrossRef]
- Saleh, S.M. ZnO nanospheres based simple hydrothermal route for photocatalytic degradation of azo dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 141–147. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.; Meng, Y.; Liu, Y. A two-step route to synthesize highly oriented ZnO nanotube arrays. Ceram. Int. 2012, 38, 4555–4559. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, A.; Ji, X.; Zhu, Y.; Gui, X.; Huang, F.; Tang, Z. Growth of vertically aligned ZnO nanowire arrays on ZnO single crystals. Mater. Lett. 2015, 154, 40–43. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Kim, G.S.; Khang, G.; Shin, H.-S. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 2007, 42, 1640–1648. [Google Scholar] [CrossRef]
- Moharram, A.H.; Mansour, S.A.; Hussein, M.A.; Rashad, M. Direct Precipitation and Characterization of ZnO Nanoparticles. J. Nanomater. 2014, 2014, 716210. [Google Scholar] [CrossRef] [Green Version]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zeng, H.C. Hydrothermal Synthesis of ZnO Nanorods in the Diameter Regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. CrystEngComm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted Synthesis of Zinc Oxide Nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Taunk, P.B.; Das, R.; Bisen, D.P.; Tamrakar, R.K.; Rathor, N. Synthesis and optical properties of chemical bath deposited ZnO thin film. Karbala Int. J. Mod. Sci. 2015, 1, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Blachowicz, T.; Ehrmann, A. Recent developments in electrospun ZnO nanofibers: A short review. J. Eng. Fibers Fabr. 2020, 15, 1558925019899682. [Google Scholar] [CrossRef] [Green Version]
- Illy, B.N.; Cruickshank, A.C.; Schumann, S.; Da Campo, R.; Jones, T.S.; Heutz, S.; McLachlan, M.A.; McComb, D.W.; Riley, D.J.; Ryan, M.P. Electrodeposition of ZnO layers for photovoltaic applications: Controlling film thickness and orientation. J. Mater. Chem. 2011, 21, 12949–12957. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z. ZnO thin films produced by magnetron sputtering. Ceram. Int. 2004, 30, 1155–1159. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Martins, R.F.P.; Pereira, L.M.N. Wide-bandgap high-mobility ZnO thin-film transistors produced at room temperature. Appl. Phys. Lett. 2004, 85, 2541–2543. [Google Scholar] [CrossRef]
- Golshahi, S. P-type ZnO thin film deposited by spray pyrolysis technique: The effect of solution concentration. Thin Solid Film. 2009, 518, 1149–1152. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef]
- Araújo, A.; Pimentel, A.; Oliveira, M.J.; Mendes, M.J.; Franco, R.; Fortunato, E.; Águas, H.; Martins, R. Direct growth of plasmonic nanorod forests on paper substrates for low-cost flexible 3D SERS platforms. Flex. Print. Electron. 2017, 2, 014001. [Google Scholar] [CrossRef]
- Manekkathodi, A.; Lu, M.-Y.; Wang, C.W.; Chen, L.-J. Direct Growth of Aligned Zinc Oxide Nanorods on Paper Substrates for Low-Cost Flexible Electronics. Adv. Mater. 2010, 22, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Ghule, K.; Ghule, A.V.; Chen, B.-J.; Ling, Y.-C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Zhang, X.; Weng, J.; Zhang, J.; Zhang, M. Protective coating of paper works: ZnO/cellulose nanocrystal composites and analytical characterization. J. Cult. Herit. 2019, 38, 64–74. [Google Scholar] [CrossRef]
- Zhao, S.-W.; Guo, C.-R.; Hu, Y.-Z.; Guo, Y.-R.; Pan, Q.-J. The preparation and antibacterial activity of cellulose/ZnO composite: A review. Open Chem. 2018, 16, 9–20. [Google Scholar] [CrossRef]
- Shao, D.; Sun, H.; Gao, J.; Xin, G.; Anthony Aguilar, M.; Yao, T.; Koratkar, N.; Lian, J.; Sawyer, S. Flexible, thorn-like ZnO-multiwalled carbon nanotube hybrid paper for efficient ultraviolet sensing and photocatalyst applications. Nanoscale 2014, 6, 13630–13636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, K.; Wu, J.; Gu, L.; Lu, Z.; Wang, X.; Cao, X. Synthesis of highly permeable Fe2O3/ZnO hollow spheres for printable photocatalysis. RSC Adv. 2015, 5, 88277–88286. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W. Cellulose-based photocatalytic paper with Ag2O nanoparticles loaded on graphite fibers. J. Bioresour. Bioprod. 2016, 1, 192–198. [Google Scholar]
- Hua, C.; Liu, X.; Ren, S.; Zhang, C.; Liu, W. Preparation of visible light-responsive photocatalytic paper containing BiVO4@diatomite/MCC/PVBCFs for degradation of organic pollutants. Ecotoxicol. Environ. Saf. 2020, 202, 110897. [Google Scholar] [CrossRef] [PubMed]
- ISO10678:2010. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. Available online: https://www.iso.org/standard/46019.html (accessed on 20 February 2021).
- Shi, Z.; Zang, S.; Jiang, F.; Huang, L.; Lu, D.; Ma, Y.; Yang, G. In situ nano-assembly of bacterial cellulose–polyaniline composites. RSC Adv. 2012, 2, 1040–1046. [Google Scholar] [CrossRef]
- Saito, Y.; Kamitakahara, H.; Takano, T. Preparation of a squaraine-bounded cellulose derivative for photocurrent generation system. Carbohydr. Res. 2016, 421, 40–45. [Google Scholar] [CrossRef]












| Material | Target Pollutant/Medium | Light Source | Reference |
|---|---|---|---|
| Paper with TiO2 aggregates | Acetaldehyde in gas | Weak UV | [180] |
| Paper with TiO2 supported on inorganic fibers | Acetaldehyde in gas | UV | [181] |
| Paper with nano TiO2 powders supported on X zeolite | Acetaldehyde in gas | VIS | [182] |
| Papers containing TiO2 decorated with AgBr nanoparticles | 2-propanol in gas | Sunlight | [183] |
| Paper with TiO2 nanoparticles confined in hollow silica capsules | 2-propanol in gas | UV | [55] |
| Cellulose-based material with TiO2 nanoparticles loaded on carbon fibers | Methyl orange in liquid | UV | [57] |
| Papers containing poly-dopamine-loaded cellulose fiere together with pristine cellulose fiere and TiO2 nanoparticles | Methyl orange in liquid | UV | [58] |
| TiO2-coated non-woven paper with colloidal SiO2 binder | Rhodamine B in liquid | UV | [184] |
| Paper with TiO2 nanosol | Blue indigo carmine in liquid | UV | [186] |
| TiO2 hydrosols on paper sheets | Methylene blue in liquid | UV | [187] |
| Paper based on a TiO2/Sodium alginate nanocomposite | Chemical oxygen demand of wastewater/liquid | UV | [185] |
| Paper-TiO2 composite | 2-propanol in gas | Sunlight | [178] |
| Au nanoparticles decorating TiO2 nanowires onto hierarchically porous carbon fiber paper | Styrene in gas | VIS | [177] |
| Papers composed by nanosized TiO2 supported on natural zeolite | Toluene in gas | UV | [188,189] |
| Paper formed by a composite of TiO2/SiO2 particles | Ethanol in gas | UV | [190] |
| BNC with TiO2nanostructured films | Rhodamine B in liquid | Sunlight | [19] |
| Papers with TiO2, ZnO and ZnO/ TiO2 nanostructured films | Rhodamine B in liquid | UV and sunlight | [12] |
| Papers with TiO2 thin films | Rhodamine B in liquid | Sunlight | [192] |
| Paper with ZnO nanorods | Methylene blue and methyl orange in liquid | VIS | [20] |
| Paper with Cu2O and Ag nanoparticles decorating ZnO nanorods | Rhodamine B in liquid | VIS | [54] |
| Thorn-like ZnO-multiwalled carbon nanotube hybrid paper | Rhodamine B in liquid | UV | [228] |
| Papers with Fe2O3/ZnO hollow spheres | 2,4,6-trichlorophenol in liquid | Sunlight | [229] |
| Paper containing Ag2O nanoparticles | Methyl orange in liquid | UV, VIS or near-IR | [230] |
| Paper based of BiVO4@diatomite/microcrystalline cellulose/ poly(vinyl butyral) | Methylene blue in liquid and formaldehyde in gas | VIS | [231] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation. Catalysts 2021, 11, 504. https://doi.org/10.3390/catal11040504
Nunes D, Pimentel A, Branquinho R, Fortunato E, Martins R. Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation. Catalysts. 2021; 11(4):504. https://doi.org/10.3390/catal11040504
Chicago/Turabian StyleNunes, Daniela, Ana Pimentel, Rita Branquinho, Elvira Fortunato, and Rodrigo Martins. 2021. "Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation" Catalysts 11, no. 4: 504. https://doi.org/10.3390/catal11040504