Nickel-Fe3O4 Magnetic Nanoparticles Supported on Multiwalled Carbon Nanotubes: Effective Catalyst in Suzuki Cross Coupling Reactions
Abstract
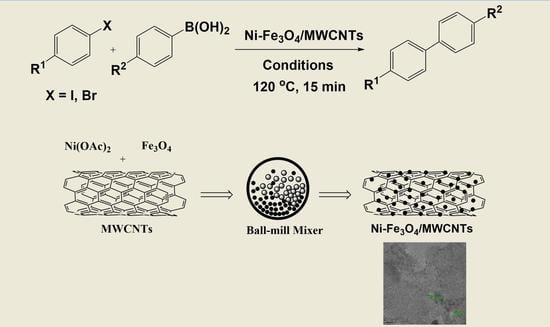
:1. Introduction
2. Results and Discussion
2.1. Application in Suzuki Cross Coupling Reactions
2.2. Recycling of Ni-Fe3O4/MWCNTs
2.3. Diversity of Substrates in Suzuki Cross Coupling Reactions
3. Experimental
3.1. General Methods
3.2. Synthesis of Ni-Fe3O4/MWCNTs Nanoparticles
3.3. Procedure for Recycling the Catalyst
3.4. Procedure for Suzuki Cross-Coupling Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandsma, L.; Verkruijsse, H.D.; Vasilevsk, S.F. Application of Transition Metal Catalysts in Organic Synthesis; Springer: Berlin/Heidelberg, Germany, 1999; Print ISBN: 978-3-540-65550-3; Online ISBN: 978-3-642-60328-0. [Google Scholar]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef]
- Carin, C.C.; Seechurn, J.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Stille, J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles [New Synthetic Methods (58)]. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent advances in nickel catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Ananikov, V.P. Nickel: The “Spirited Horse” of transition metal catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef] [Green Version]
- Diccianni, J.B.; Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar] [CrossRef]
- Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef]
- Richardson, J.M.; Jones, C.W. Leached nickel promotes catalysis using supported Ni (II) complex precatalysts in Kumada-Corriu reactions. J. Mol. Catal. A Chem. 2009, 297, 125–134. [Google Scholar] [CrossRef]
- Netherton, M.R.; Fu, G.C. Nickel-catalyzed cross-couplings of unactivated alkyl halides and pseudohalides with organometallic compounds. Adv. Synth. Catal. 2004, 346, 1525–1532. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L. Recent advances in mechanistic studies on Ni catalyzed cross-coupling reactions. Chin. J. Catal. 2015, 36, 3–14. [Google Scholar] [CrossRef]
- Weber, J.M.; Longstreet, A.R.; Jamison, T.F. Bench-stable nickel precatalysts with heck-type activation. Organometallics 2018, 37, 2716–2722. [Google Scholar] [CrossRef]
- Costa, N.J.S.; Guerrero, M.; Collière, V.; Teixeira-Neto, É.; Landers, R.; Philippot, K.; Rossi, L.M. Organometallic preparation of Ni, Pd, and NiPd nanoparticles for the design of supported nanocatalysts. ACS Catal. 2014, 4, 1735–1742. [Google Scholar] [CrossRef]
- Lundgren, R.J.; Stradiotto, M. Addressing challenges in palladium-catalyzed cross-coupling reactions through ligand design. Chem. A Eur. J. 2012, 18, 9758–9769. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Arai, M. Catalyst product separation techniques in heck reaction. Catal. Rev. 2001, 43, 315–344. [Google Scholar] [CrossRef]
- Köhler, K.; Heidenreich, R.G.; Soomro, S.S.; Pröckl, S.S. Supported Palladium Catalysts for Suzuki Reactions: Structure-Property Relationships, Optimized Reaction Protocol and Control of Palladium Leaching. Adv. Synth. Catal. 2008, 350, 2930. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K. Heck reaction catalyzed by Pd-modified zeolites. J. Am. Chem. Soc. 2001, 123, 5990–5999. [Google Scholar] [CrossRef] [PubMed]
- Djakovitch, L.; Koehler, K. Heck reactions between aryl halides and olefins catalysed by Pd-complexes entrapped into zeolites. N. Y. J. Mol. Catal. A Chem. 1999, 142, 275. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Zhao, F.-G.; Shirai, M.; Arai, M. Comparison of activity and selectivity of various metal-TPPTS complex catalysts in ethylene glycol toluene biphasic Heck vi-nylation reactions of iodobenzene. Tetrahedron Lett. 1998, 39, 9509–9512. [Google Scholar] [CrossRef]
- Erdoğan, H.; Metin, Ö.; Özkar, S. In situ-generated PVP-stabilized palladium (0) nanocluster catalyst in hydrogen generation from the methanolysis of ammonia–borane. Phys. Chem. Chem. Phys. 2009, 11, 10519. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, C.; Saha, I.; Zheng, Y.; Goodson, B.M.; Gao, Y. Dendron-Functionalized Superparamagnetic Nanoparticles with Switchable Solubility in Organic and Aqueous Media: Matrices for Homogeneous Catalysis and Potential MRI Contrast Agents. Chem. Mater. 2006, 18, 5973. [Google Scholar] [CrossRef]
- El Hankari, S.; El Kadib, A.; Finiels, A.; Bouhaouss, A.; Moreau, J.J.E.; Crudden, C.M.; Brunel, D.; Hesemann, P. SBA-15-Type Organosilica with 4-Mercapto-N,N-bis-(3-Si-propyl)butanamide for Palladium Scavenging and Cross-Coupling Catalysis. Chem. Eur. J. 2011, 17, 8984. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Effect of colloidal catalysis on the nanoparticle size distribution: Dendrimer−Pd vs PVP−Pd Nanoparticles catalyzing the Suzuki coupling reaction. J. Phys. Chem. B 2004, 108, 8572–8580. [Google Scholar] [CrossRef]
- Ellis, P.J.; Fairlamb, I.J.S.; Hackett, S.F.J.; Wilson, K.; Lee, A.F. Evidence for the surface-catalyzed Suzuki-Miyaura reaction over palladium nanoparticles: An operando XAS study. Angew. Chem. Int. Ed. 2010, 49, 1820–1824. [Google Scholar] [CrossRef]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mülhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki−Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Stroscio, M.A.; Dutta, M. Thermal conductivity of carbon nanotubes. J. Appl. Phys. 2009, 105, 74316. [Google Scholar] [CrossRef]
- Che, J.; Cagin, T.; Goddard, W.A. III thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Cat. A General 2004, 35, 337. [Google Scholar] [CrossRef]
- Serp, P.; Castillejos, E. Catalysis in carbon nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Cornelio, B.; Rance, G.A.; Laronze-Cochard, M.; Fontana, A.; Sapi, J.; Khlobystov, A. Palladium nanoparticles on carbon nanotubes as catalysts of cross-coupling reactions. J. Mater. Chem. A 2013, 1, 8737. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon-carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Chun, Y.S.; Shin, J.Y.; Song, C.E.; Lee, S.-G. Palladium nanoparticles supported onto ionic carbon nanotubes as robust recyclable catalysts in an ionic liquid. Chem. Commun. 2008, 8, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Jang, Y.; Park, J.; Na, H.B.; Park, H.M.; Yun, H.J.; Lee, J.; Hyeon, T. Designed synthesis of atom-economical Pd/Ni bimetallic nanoparticle-based catalysts for sonogashira coupling reactions. J. Am. Chem. Soc. 2004, 126, 5026–5027. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Lin, Y.; Baggett, D.W.; Kim, J.-W.; Siochi, E.J.; Connell, J.W. Instantaneous formation of metal and metal oxide nanoparticles on carbon nanotubes and graphene via solvent-free microwave heating. ACS Appl. Mater. Interfaces 2011, 3, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Landge, S.; Ghosh, D.; Aiken, K. Solvent-free synthesis of nanoparticles. Green Chem. 2018, 609–646. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free Suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Xie, D.; Chen, G. Polyaniline-coated Fe3O4 nanoparticle-carbon-nanotube composite and its application in electrochemical biosensing. Small 2008, 4, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, Z.; Zhang, Y.; Wang, R.; Luo, S.-Z.; Jing, F.; Chu, W. Carbon nanotubes supported nickel as the highly efficient catalyst for hydrogen production through glycerol steam reforming. ACS Sustain. Chem. Eng. 2018, 6, 14403–14413. [Google Scholar] [CrossRef]
- Gonzalez, I.; De Jesus, J.C.; Cañizales, E.; Delgado, B.; Urbina, C. Comparison of the surface state of Ni nanoparticles used for methane catalytic decomposition. J. Phys. Chem. C 2012, 116, 21577–21587. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of nanoscaled and bulk NiO structural and environmental characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Li, J.; Tian, Z.; Yu, F. Highly-dispersed Ni-NiO nanoparticles anchored on an SiO2 support for an enhanced CO methanation performance. Catalysts 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; De Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situXPS analysis of various iron oxide films grown byNO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Obermayer, D.; Znidar, D.; Glotz, G.; Stadler, A.; Dallinger, D.; Kappe, C.O. Design and performance validation of a conductively heated sealed-vessel reactor for organic synthesis. J. Org. Chem. 2016, 81, 11788–11801. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.; Cameron, T.S.; Jha, M. Bronsted acid-catalyzed rapid enol-ether formation of 2-hydroxyindole-3-carboxaldehydes. Mol. Divers. 2013, 17, 827–834. [Google Scholar] [CrossRef]
- Jha, M.; Edmunds, M.; Lund, K.-L.; Ryan, A. A new route to the versatile synthesis of thiopyrano [2,3-b:6,5-b′] diindoles via 2-(alkylthio)-indole-3-carbaldehydes. Tetrahedron Lett. 2014, 55, 5691–5694. [Google Scholar] [CrossRef]
- Oliverio, M.; Nardi, M.; Costanzo, P.; Cariati, L.; Cravotto, G.; Giofrè, S.V.; Procopio, A. Non-conventional methodologies in the synthesis of 1-Indanones. Molecules 2014, 19, 5599–5610. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Entry | Cat Loading (mol%) | Solvent | Base | Temp (oC) | Time (Min) | Conversion % b |
|---|---|---|---|---|---|---|
| 1 | 3 | DMF | K2CO3 | 120 | 15 | 10 |
| 2 | 3 | DMF | NEt3 | 120 | 15 | 15 |
| 3 | 3 | H2O | K2CO3 | 120 | 15 | 0 |
| 4 | 3 | H2O:EtOH | NEt3 | 120 | 15 | 45 |
| 5 | 3 | H2O:EtOH | Cs2CO3 | 120 | 15 | 80 |
| 6 | 3 | H2O:EtOH | K2CO3 | 100 | 15 | 85 |
| 7 | 3 | H2O:EtOH | K2CO3 | 120 | 15 | 100 |
| 8 | 3 | H2O:EtOH | K2CO3 | 120 | 10 | 90 |
| 9 | 1 | H2O:EtOH | K2CO3 | 120 | 15 | 100 |
| 10 | 0.5 | H2O:EtOH | K2CO3 | 120 | 15 | 100 |
| 11 | 0.05 | H2O:EtOH | K2CO3 | 120 | 15 | 100 |
| 12 | 0.005 | H2O:EtOH | K2CO3 | 120 | 15 | 95 |
| 13 c | - | H2O:EtOH | K2CO3 | 120 | 15 | 0 |
| 14 d | 3 | H2O:EtOH | K2CO3 | 120 | 15 | 0 |
| 15 e | 3 | H2O:EtOH | K2CO3 | 120 | 15 | 0 |
| Run | Conversion % b |
|---|---|
| 1 | 100 |
| 2 | 100 |
| 3 | 100 |
| 4 | 100 |
| 5 | 100 |
| 6 | 100 |
| 7 | 95 |
| 8 | 95 |
| 9 | 95 |

| cpd | Aryl-Halide | Boronic Acid | 1(%) b |
|---|---|---|---|
| a |  |  |  |
| b |  |  |  |
| c |  |  |  |
| d |  |  |  |
| e |  |  |  |
| f |  |  |  |
| g |  |  |  |
| h |  |  |  |
| i |  |  |  |
| j |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folsom, S.K.; Ivey, D.J.; McNair, F.S.; Siamaki, A.R. Nickel-Fe3O4 Magnetic Nanoparticles Supported on Multiwalled Carbon Nanotubes: Effective Catalyst in Suzuki Cross Coupling Reactions. Catalysts 2021, 11, 495. https://doi.org/10.3390/catal11040495
Folsom SK, Ivey DJ, McNair FS, Siamaki AR. Nickel-Fe3O4 Magnetic Nanoparticles Supported on Multiwalled Carbon Nanotubes: Effective Catalyst in Suzuki Cross Coupling Reactions. Catalysts. 2021; 11(4):495. https://doi.org/10.3390/catal11040495
Chicago/Turabian StyleFolsom, Sojeong K., Destiny J. Ivey, Frank S. McNair, and Ali R. Siamaki. 2021. "Nickel-Fe3O4 Magnetic Nanoparticles Supported on Multiwalled Carbon Nanotubes: Effective Catalyst in Suzuki Cross Coupling Reactions" Catalysts 11, no. 4: 495. https://doi.org/10.3390/catal11040495