Enhanced Activity of Titanocene Complex for Electrocatalytic Nitrogen Reduction Reaction
Abstract
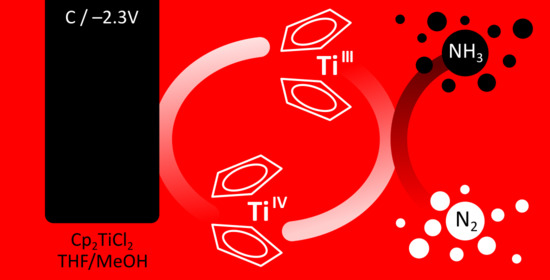
:1. Introduction
2. Results and Discussion
3. Experimental
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef] [PubMed]
- Bosch, C. Process of Producing Ammonia. U.S. Patent 990191, 18 April 1911. [Google Scholar]
- Haber, F.; Le Rossignol, R. Über die technische Darstellung von Ammoniak aus den Elementen. Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie 1913, 19, 53–72. [Google Scholar]
- Van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, J.; Vourros, A.; Vasileiou, E.; Stoukides, M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Birol, F. The Future of Hydrogen. Seizing Today’s Opportunities. Report Prepared by the IEA for the G20, Japan; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef]
- Milton, R.D.; Abdellaoui, S.; Khadka, N.; Dean, D.R.; Leech, D.; Seefeldt, L.C.; Minteer, S.D. Nitrogenase bioelectrocatalysis: Heterogeneous ammonia and hydrogen production by MoFe protein. Energy Environ. Sci. 2016, 9, 2550–2554. [Google Scholar] [CrossRef] [Green Version]
- Milton, R.D.; Minteer, S.D. Enzymatic Bioelectrosynthetic Ammonia Production: Recent Electrochemistry of Nitrogenase, Nitrate Reductase, and Nitrite Reductase. ChemPlusChem 2017, 82, 513–521. [Google Scholar] [CrossRef]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Cai, R.; Abdellaoui, S.; Leech, D.; De Lacey, A.L.; Pita, M.; Minteer, S.D. Bioelectrochemical Haber-Bosch Process: An Ammonia-Producing H2 /N2 Fuel Cell. Angew. Chem. Int. Ed. 2017, 56, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Fourmond, V.; Léger, C. Dinitrogen Reduction: Interfacing the Enzyme Nitrogenase with Electrodes. Angew. Chem. Int. Ed. 2017, 56, 4388–4390. [Google Scholar] [CrossRef] [PubMed]
- Vol’pin, M.E.; Shur, V.B. Nitrogen fixation on complex catalysts. Doklady Akademii Nauk SSSR 1964, 156, 1102–1104. [Google Scholar]
- Shilov, A.E.; Shilova, A.K.; Kvashina, E.F. Kinet. Katal. 1969, 10, 1402. [Google Scholar]
- Chatt, J.; Pearman, A.J.; Richards, R.L. The reduction of mono-coordinated molecular nitrogen to ammonia in a protic environment. Nature 1975, 253, 39–40. [Google Scholar] [CrossRef]
- Hidai, M.; Takahashi, T.; Yokotake, I.; Uchida, Y. Reactions Of Ligating Dinitrogen With Alcohols Or Metal Hydrides. Chem. Lett. 1980, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Yandulov, D.V.; Schrock, R.R. Catalytic Reduction of Dinitrogen to Ammonia at a Single Molybdenum Center. Science 2003, 301, 76–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.S.; Rittle, J.; Peters, J.C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 2013, 501, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Creutz, S.E.; Peters, J.C. Catalytic Reduction of N2 to NH3 by an Fe–N2 Complex Featuring a C-Atom Anchor. J. Am. Chem. Soc. 2014, 136, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.S.; Cutsail, G.E.; Rittle, J.; Connor, B.A.; Gunderson, W.A.; Zhang, L.; Hoffman, B.M.; Peters, J.C. Characterization of an Fe≡N–NH2 Intermediate Relevant to Catalytic N2 Reduction to NH3. J. Am. Chem. Soc. 2015, 137, 7803–7809. [Google Scholar] [CrossRef] [Green Version]
- Rittle, J.; Peters, J.C. An Fe-N2 Complex That Generates Hydrazine and Ammonia via Fe=NNH2: Demonstrating a Hybrid Distal-to-Alternating Pathway for N2 Reduction. J. Am. Chem. Soc. 2016, 138, 4243–4248. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, T.J.; Thompson, N.B.; Peters, J.C. A Synthetic Single-Site Fe Nitrogenase: High Turnover, Freeze-Quench 57Fe Mössbauer Data, and a Hydride Resting State. J. Am. Chem. Soc. 2016, 138, 5341–5350. [Google Scholar] [CrossRef] [Green Version]
- Chalkley, M.J.; Del Castillo, T.J.; Matson, B.D.; Roddy, J.P.; Peters, J.C. Catalytic N2-to-NH3 Conversion by Fe at Lower Driving Force: A Proposed Role for Metallocene-Mediated PCET. ACS Cent. Sci. 2017, 3, 217–223. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Photolysis of aqueous [Os(NH3)5(N2)]2+: Dismutation of coordinated dinitrogen. Inorganica Chim. Acta 2012, 391, 229–231. [Google Scholar] [CrossRef]
- Banerjee, A.; Yuhas, B.D.; Margulies, E.A.; Zhang, Y.; Shim, Y.; Wasielewski, M.R.; Kanatzidis, M.G. Photochemical Nitrogen Conversion to Ammonia in Ambient Conditions with FeMoS-Chalcogels. J. Am. Chem. Soc. 2015, 137, 2030–2034. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Li, J.F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef]
- Lv, C.; Yan, C.; Chen, G.; Ding, Y.; Sun, J.; Zhou, Y.; Yu, G. An Amorphous Noble-Metal-Free Electrocatalyst that Enables Nitrogen Fixation under Ambient Conditions. Angew. Chem. Int. Ed. 2018, 57, 6073–6076. [Google Scholar] [CrossRef]
- Lv, C.; Qian, Y.; Yan, C.; Ding, Y.; Liu, Y.; Chen, G.; Yu, G. Defect Engineering Metal-Free Polymeric Carbon Nitride Electrocatalyst for Effective Nitrogen Fixation under Ambient Conditions. Angew. Chem. Int. Ed. 2018, 57, 10246–10250. [Google Scholar] [CrossRef]
- Bayer, E.; Schurig, V. Stickstoff-Fixierung und Reduktion zu Ammoniak mit metall-organischen Katalysatoren. Chem. Ber. 1969, 102, 3378–3390. [Google Scholar] [CrossRef]
- Van Tamelen, E.E.; Fechter, R.B.; Schneller, S.W.; Boche, G.; Greeley, R.H.; Akermark, B. Titanium(II) in the fixation-reduction of molecular nitrogen under mild conditions. J. Am. Chem. Soc. 1969, 91, 1551–1552. [Google Scholar] [CrossRef]
- Van Tamelen, E.E.; Seeley, D.A. The Catalytic Fixation of Molecular Nitrogen by Electronic and Chemical Reduction. J. Am. Chem. Soc. 1969, 91, 5194. [Google Scholar] [CrossRef]
- Becker, J.Y.; Avraham, S.; Posin, B. Part I. Electrochemical reduction of titanium compounds in the presence of catechol and N2 in MeOH or THF. J. Electroanal. Chem. 1987, 230, 143–153. [Google Scholar] [CrossRef]
- Battino, R.; Rettich, T.R.; Tominaga, T. The Solubility of Nitrogen and Air in Liquids. J. Phys. Chem. Ref. Data 1984, 13, 563. [Google Scholar] [CrossRef] [Green Version]
- Panda, H. The Complete Book on Electroplating & Allied Chemicals; Asia Pacific Business Press: New Delhi, India, 2013; p. 204. [Google Scholar]





| Electrolyte | n-Bu4NBF4 | LiClO4 | n-Bu4NClO4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechol | No | 10 eq. | No | 10 eq. | No | 10 eq. | ||||||
| Atmosphere | Ar | N2 | Ar | N2 | Ar | N2 | Ar | N2 | Ar | N2 | Ar | N2 |
| MeCN | – | – | – | – | – | – | – | – | – | – | – | – |
| MeOH | – | – | – | – | – | – | – | – | – | – | – | + |
| THF | – | – | – | – | – | – | – | – | – | + | – | ++ |
| THF/MeOH 9:1 | – | – | – | – | – | – | – | ++ | – | ++ | – | +++ |
| Solvent | Electrode | E (V) | Time (h) | NH3 (µg) | TON (%) | Activity |
|---|---|---|---|---|---|---|
| THF/MeOH | C | −1.5 | 24.0 | 0.46 | 0.14 | 0.0054 |
| THF/MeOH | C | −1.6 | 24.0 | 0.54 | 0.16 | 0.0063 |
| THF/MeOH | C | −1.8 | 24.0 | 3.82 | 1.12 | 0.0449 |
| THF/MeOH | C | −1.9 | 24.0 | 4.40 | 1.29 | 0.0517 |
| THF/MeOH | C | −2.0 | 24.0 | 5.36 | 1.57 | 0.0629 |
| THF/MeOH | C | −2.3 | 24.0 | 9.64 | 2.83 | 0.1132 |
| MeOH | Hg | −1.2 | 4.33 | 28.1 * | 0.06 * | 0.0022 * |
| MeOH | Hg | −2.2 | 0.50 | 45.3 * | 0.09 * | 0.0036 * |
| MeOH | Pt | −1.2 | 5.00 | 340.6 * | 0.67 * | 0.0268 * |
| MeOH | Pt | −2.2 | 4.83 | 744.3 *§ | 1.45 *§ | 0.0585 *§ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fijalkowski, K.J. Enhanced Activity of Titanocene Complex for Electrocatalytic Nitrogen Reduction Reaction. Catalysts 2021, 11, 389. https://doi.org/10.3390/catal11030389
Fijalkowski KJ. Enhanced Activity of Titanocene Complex for Electrocatalytic Nitrogen Reduction Reaction. Catalysts. 2021; 11(3):389. https://doi.org/10.3390/catal11030389
Chicago/Turabian StyleFijalkowski, Karol J. 2021. "Enhanced Activity of Titanocene Complex for Electrocatalytic Nitrogen Reduction Reaction" Catalysts 11, no. 3: 389. https://doi.org/10.3390/catal11030389