Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ethanol Dry Reforming Catalyzed by Ni/Al2O3
2.2. Characterizations of Alkali Metal Promoted Ni/Al2O3
2.3. Ethanol Dry Reforming Catalyzed by Alkali Metal Modified Ni/Al2O3
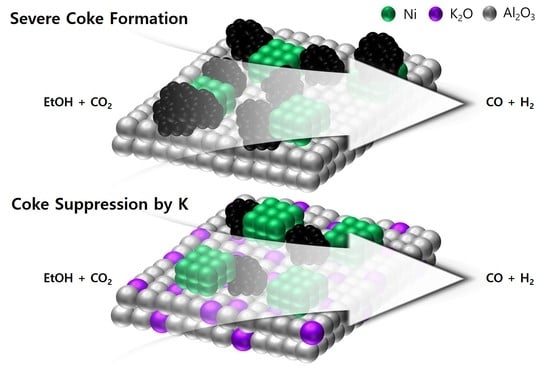
2.4. Characterizations of Deposited Coke
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Ethanol Dry Reforming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubb, M.; Vrolijk, C.; Brack, D. Kyoto Protocol: A Guide and Assessment, 1st ed.; Routledge: London, UK, 1999. [Google Scholar]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Qu, J.; Su, A.; Zhang, Y.; Xu, Y. Towards understanding the carbon catalyzed CO2 reforming of methane to syngas. J. Ind. Eng. Chem. 2015, 21, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.H. The influence of global warming on natural disasters and their public health outcomes. Am. J. Disaster Med. 2007, 2, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef] [Green Version]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cui, H.; Wei, S.; Zhuo, S.; Wang, L.; Li, Z.; Yi, W. Separation of biomass pyrolysis oil by supercritical CO2 extraction. Smart Grid Renew. Energy 2010, 1, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam reforming of ethanol on Ni/CeO2: Reaction pathway and interaction between Ni and the CeO2 support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.M. CO2 reforming of methane on Ni catalysts: Effects of the support phase and preparation technique. Appl. Catal. B 1998, 16, 269–277. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Syngas production by CO2 reforming of ethanol over Ni/Al2O3 catalyst. Catal. Commun. 2009, 10, 1633–1637. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Shi, Z. Nickel carbonyl: Toxicity and human health. Sci. Total. Environ. 1994, 148, 293–298. [Google Scholar] [CrossRef]
- Bellido, J.D.A.; Tanabe, E.Y.; Assaf, E.M. Carbon dioxide reforming of ethanol over Ni/Y2O3–ZrO2 catalysts. Appl. Catal. B 2009, 90, 485–488. [Google Scholar] [CrossRef]
- Bahari, M.B.; Phuc, N.H.H.; Alenazey, F.; Vu, K.B.; Ainirazali, N.; Vo, D.-V.N. Catalytic performance of La-Ni/Al2O3 catalyst for CO2 reforming of ethanol. Catal. Today 2017, 291, 67–75. [Google Scholar] [CrossRef]
- Bahari, M.; Fayaz, F.; Ainirazali, N.; Huy, P.N.H.; Vo, D.-V.N. Evaluation of co-promoted Ni/Al2O3 catalyst for CO2 reforming of ethanol. J. Eng. Applied Sci. 2016, 11, 7249–7253. [Google Scholar]
- Cao, D.; Zeng, F.; Zhao, Z.; Cai, W.; Li, Y.; Yu, H.; Zhang, S.; Qu, F. Cu based catalysts for syngas production from ethanol dry reforming: Effect of oxide supports. Fuel 2018, 219, 406–416. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.-V.N. Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: A short review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- De Souza, G.; Balzaretti, N.; Marcilio, N.; Perez-Lopez, O. Decomposition of ethanol over Ni-Al catalysts: Effect of copper addition. Procedia Eng. 2012, 42, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Sivaramakrishnan, R.; Su, M.-C.; Michael, J.V.; Klippenstein, S.J.; Harding, L.B.; Ruscic, B. Rate constants for the thermal decomposition of ethanol and its bimolecular reactions with OH and D: Reflected shock tube and theoretical studies. J. Phys. Chem. A 2010, 114, 9425–9439. [Google Scholar] [CrossRef]
- Xu, G.; Shi, K.; Gao, Y.; Xu, H.; Wei, Y. Studies of reforming natural gas with carbon dioxide to produce synthesis gas: X. The role of CeO2 and MgO promoters. J. Mol. Catal. A 1999, 147, 47–54. [Google Scholar] [CrossRef]
- Nederlof, C.; Talay, G.; Kapteijn, F.; Makkee, M. The role of RWGS in the dehydrogenation of ethylbenzene to styrene in CO2. Appl. Catal. A 2012, 59–68. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; El Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B 2017, 201, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; An, H.; Tan, E. Screening of CO2 adsorbing materials for zero emission power generation systems. Energy Fuels 2007, 21, 426–434. [Google Scholar] [CrossRef]
- Lu, H.; Reddy, E.P.; Smirniotis, P.G. Calcium oxide based sorbents for capture of carbon dioxide at high temperatures. Ind. Eng. Chem. Res. 2006, 45, 3944–3949. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Effects of promoters on catalytic activity and carbon deposition of Ni/γ-Al2O3 catalysts in CO2 reforming of CH4. J. Chem. Tech. Biotech. 2000, 75, 589–595. [Google Scholar] [CrossRef]
- Pegios, N.; Bliznuk, V.; Prünte, S.; Schneider, J.M.; Palkovits, R.; Simeonov, K. Comparative study on La-promoted Ni/γ-Al2O3 for methane dry reforming—Spray drying for enhanced nickel dispersion and strong metal—Support interactions. RSC Adv. 2018, 8, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Zangouei, M.; Moghaddam, A.Z.; Arasteh, M. The influence of nickel loading on reducibility of NiO/Al2O3 catalysts synthesized by sol-gel method. Chem. Eng. Res. Bull. 2010, 14, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Alberton, A.L.; Souza, M.M.; Schmal, M. Carbon formation and its influence on ethanol steam reforming over Ni/Al2O3 catalysts. Catal. Today 2007, 123, 257–264. [Google Scholar] [CrossRef]
- Kim, P.; Joo, J.; Kim, H.; Kim, W.; Kim, Y.; Song, I.; Yi, J. Preparation of mesoporous Ni–alumina catalyst by one-step Sol–gel method: Control of textural properties and catalytic application to the hydrodechlorination of o-dichlorobenzene. Catal. Lett. 2005, 104, 181–189. [Google Scholar] [CrossRef]
- Huang, L.Q.; Zhang, Z.H.; Guo, Z.L. The effect of MgO precoating on the perfomance of Ni/MgO-Al2O3 catalysts for carbon dioxide reforming of methane. Adv. Mater. Res. 2014, 997, 272–278. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Marton, M.; Vojs, M.; Zdravecká, E.; Himmerlich, M.; Haensel, T.; Krischok, S.; Kotlar, M.; Michniak, P.; Vesely, M.; Redhammer, R. Raman spectroscopy of amorphous carbon prepared by pulsed arc discharge in various gas mixtures. J. Spectrosc. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Ge, Q.; Xu, H.; Li, W. Effects of Ce addition on the Pt-Sn/γ-Al2O3 catalyst for propane dehydrogenation to propylene. Appl. Catal. A 2006, 315, 58–67. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over catalytic surfaces. Chem. Phys. Chem. 2017, 18, 3135–3141. [Google Scholar] [CrossRef] [Green Version]












| Entry | Sample | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| 1 | Al2O3 | 205 | 9 | 0.48 |
| 2 | Li2O-Al2O3 | 89 | 16 | 0.41 |
| 3 | Na2O-Al2O3 | 142 | 11 | 0.41 |
| 4 | K2O-Al2O3 | 166 | 10 | 0.41 |
| 5 | Ni/Al2O3 | 143 | 11 | 0.4 |
| 6 | Ni/Li2O-Al2O3 | 65 | 17 | 0.27 |
| 7 | Ni/Na2O-Al2O3 | 127 | 9 | 0.3 |
| 8 | Ni/K2O-Al2O3 | 137 | 9 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-W.; Lee, D.; Kim, S.-I.; Kim, Y.J.; Park, J.H.; Heo, I.; Chang, T.S.; Lee, J.H. Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming. Catalysts 2021, 11, 260. https://doi.org/10.3390/catal11020260
Park S-W, Lee D, Kim S-I, Kim YJ, Park JH, Heo I, Chang TS, Lee JH. Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming. Catalysts. 2021; 11(2):260. https://doi.org/10.3390/catal11020260
Chicago/Turabian StylePark, Se-Won, Dongseok Lee, Seung-Ik Kim, Young Jin Kim, Ji Hoon Park, Iljeong Heo, Tae Sun Chang, and Jin Hee Lee. 2021. "Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming" Catalysts 11, no. 2: 260. https://doi.org/10.3390/catal11020260