Net-Patterned Fluorine-Doped Tin Oxide to Accelerate the Electrochromic and Photocatalytic Interface Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Morphological Properties
2.2. Structral and Chemical Properties
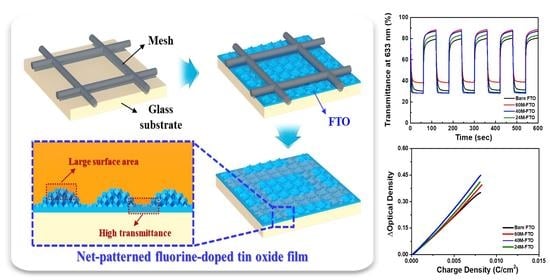
2.3. Electrochemical and Electrochromic Performance
3. Materials and Methods
3.1. Experimental Details
3.2. Characterization
3.2.1. Structures and Morphologies
3.2.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.-W.; Myoung, J.-M. Flexible and Transparent Electrochromic Displays with Simultaneously Implementable Subpixelated Ion Gel-Based Viologens by Multiple Patterning. Adv. Funct. Mater. 2019, 29, 1808911. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cong, S.; Chen, J.; Sun, H.; Chen, Z.; Song, G.; Geng, F.; Chen, Q.; Zhao, Z. Towards full-colour tunability of inorganic electrochromic devices using ultracompact fabry-perot nanocavities. Nat. Commun. 2020, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G. Transparent Conductive Electrodes for Electrochromic Devices: A Review. Appl. Phys. A 1993, 57, 19–24. [Google Scholar] [CrossRef]
- Jo, M.-H.; Koo, B.-R.; Ahn, H.-J. Accelerating F-doping in transparent conducting F-doped SnO2 films for electrochromic energy storage devices. Ceram. Int. 2020, 46, 25066–25072. [Google Scholar] [CrossRef]
- Yu, F.; Liu, W.; Ke, S.-W.; Kurmoo, M.; Zuo, J.-L.; Zhang, Q. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat. Commun. 2020, 11, 5534. [Google Scholar] [CrossRef]
- Chan, J.Y.T.; Ang, S.Y.; Ye, E.Y.; Sullivan, M.; Zhang, J.; Lin, M. Heterogeneous photo-Fenton reaction on hematite (a-Fe2O3) {104}, {113} and {001} surface facets. Phys. Chem. Chem. Phys. 2015, 17, 25333–25341. [Google Scholar] [CrossRef]
- Ye, E.; Zhang, S.-Y.; Lim, S.H.; Liu, S.; Han, M.-Y. Morphological tuning, self-assembly and optical properties of indium oxide nanocrystals. Phys. Chem. Chem. Phys. 2010, 12, 11923–11929. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.-T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Koo, B.-R.; Ahn, H.-J. Sheet resistance dependence of fluorine-doped tin oxide films for high-performance electrochromic devices. Ceram. Int. 2018, 44, 9408–9413. [Google Scholar] [CrossRef]
- Sathasivam, S.; Bhachu, D.S.; Lu, Y.; Chadwich, N.; Althabaiti, S.A.; Alyoubi, A.O.; Basahel, S.N.; Carmalt, C.J.; Parkin, I.P. Tungsten Doped TiO2 with Enhanced Photocatalytic and Optoelectrical Properties via Aerosol Assisted Chemical Vapor Deposition. Sci. Rep. 2015, 5, 10952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Zhang, S.; Qiu, C. High photocatalytic performance of high concentration Al-doped ZnO nanoparticles. Sep. Purif. Technol. 2017, 172, 236–241. [Google Scholar] [CrossRef]
- Dinh, N.N.; Quyen, N.M.; Chung, D.N.; Zikova, M.; Truong, V.-V. Highly-efficient electrochromic performance of nanostructured TiO2 films made by doctor blade technique. Sol. Energy Mater. Sol. Cells 2011, 95, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Man, W.; Yu, H.; Li, Y.; Zheng, F. Fabrication of Mo-Doped WO3 Nanorod Arrays on FTO Substrate with Enhanced Electrochromic Properties. Materials 2018, 11, 1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiamprasertboon, A.; Dixon, S.C.; Sathasivam, S.; Powell, M.J.; Lu, Y.; Siritanon, T.; Carmalt, C.J. Low-Cost One-Step Fabrication of Highly Conductive ZnO:Cl Transparent Thin Films with Tunable Photocatalytic Properties via Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Electron. Mater. 2019, 1, 1408–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaf, M.M.; Abd El–Lateef, H.M.; Ali, H.M. Optical and Photocatalytic Measurements of Co-TiO2 Nanoparticle Thin Films. Plasmonics 2018, 13, 1795–1802. [Google Scholar] [CrossRef]
- Yang, S.-H.; Yang, J.-H. Enhancement on electrochromic properties of WO3-based electrode prepared with hierarchical ZnO nanobricks. Vacuum 2020, 179, 109460. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Y.; Qiao, K.; Wei, W.; Yao, Y.; Gao, Y. of WO3/ITO nanocomposite electrochromic smart windows. Sol. Energy Mater. Sol. Cells 2019, 194, 95–102. [Google Scholar] [CrossRef]
- Wang, M.; Xing, X.; Perepichka, I.F.; Shi, Y.; Zhou, D.; Wu, P.; Meng, H. Electrochromic Smart Windows Can Achieve an Absolute Private State through Thermochromically Engineered Electrolyte. Adv. Funct. Mater. 2019, 9, 1900433. [Google Scholar] [CrossRef]
- Kim, H.; Kushto, G.P.; Auyeung, R.C.Y.; Piqué, A. Optimization of F-doped SnO2 electrodes for organic photovoltaic devices. Appl Phys. A Mater. Sci. Process. 2008, 93, 521–526. [Google Scholar] [CrossRef]
- Kim, K.-H.; Koo, B.-R.; Ahn, H.-J. Effects of Sb-doped SnO2–WO3 nanocomposite on electrochromic performance. Ceram. Int. 2019, 45, 15990–15995. [Google Scholar] [CrossRef]
- Jo, M.-H.; Koo, B.-R.; Ahn, H.-J. Fe co-doping effect on fluorine-doped tin oxide transparent conducting films accelerating electrochromic switching performance. Ceram. Int. 2020, 46, 10578–10584. [Google Scholar] [CrossRef]
- Kawashima, T.; Ezure, T.; Okada, K.; Matusi, H.; Goto, K.; Tanabe, N. FTO/ITO double layered transparent conductive oxide for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 199–202. [Google Scholar] [CrossRef]
- Lee, K.; Kim, A.-Y.; Park, J.H.; Jung, H.-G.; Choi, W.; Lee, H.Y.; Lee, J.K. Effect of micro-patterned fluorine-doped tin oxide films on electrochromic properties of Prussian blue films. Appl. Surf. Sci. 2014, 313, 864–869. [Google Scholar] [CrossRef]
- Koo, B.-R.; Oh, D.-H.; Riu, D.-H.; Ahn, H.-J. Improvement of Transparent conducting Performance on Oxygen-Activated Fluorine-Doped Tin Oxide Electrodes Formed by Horizontal Ultrasonic Spray Pyrolysis Deposition. ACS Appl. Mater. Interfaces 2017, 9, 44584–44592. [Google Scholar] [CrossRef] [PubMed]
- Chapron, D.; Girtan, M.; Le Pommelec, J.-Y.; Bouteville, A. Droplet dispersion calculations for ultrasonic spray pyrolysis depositions. J. Optoelectron. Adv. Mater. 2007, 9, 902–906. [Google Scholar]
- Perednis, D.; Gauckler, L.J. Thin Film Deposition Using Spray Pyrolysis. J. Electroceram. 2005, 14, 103–111. [Google Scholar] [CrossRef]
- Noor, N.; Parkin, I.P. Enhanced transparent-conducting fluorine-doped tin oxide films formed by aerosol-assisted chemical vapour deposition. J. Mater. Chem. C 2013, 1, 984–996. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, X.L.; Zhong, X.H.; Wang, J.N.; Pyrah, L.; Sanderson, K.D.; Ramsey, P.M.; Hirata, M.; Tsuri, K. Morphology control of fluorine-doped tin oxide thin films for enhanced light trapping. Sol. Energy Mater. Sol. Cells 2015, 132, 578–588. [Google Scholar] [CrossRef]
- Bae, J.-W.; Koo, B.-R.; An, H.-R.; Ahn, H.-J. Surface modification of fluorine-doped tin oxide films using electrochemical etching for dye-sensitized solar cells. Ceram. Int. 2015, 41, 14668–14673. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Koo, B.-R.; Ahn, H.-J. Lithium storage kinetics of highly conductive F-doped SnO2 interfacial layer on lithium manganese oxide surface. Appl. Surf. Sci. 2020, 499, 144057. [Google Scholar] [CrossRef]
- Ravichandran, K.; Ananhi, R.; Sakthivel, B.; Swaminathan, K.; Ravikumar, P.; Begum, N.J.; Snega, S. Thickness Dependence of FTO Over-Layer on Properties of FTO/FZO Bilayer. Mater. Manuf. Process. 2013, 28, 1322–1326. [Google Scholar] [CrossRef]
- Wang, Y.D.; Djerdj, I.; Antonietti, M.; Smarsly, B. Polymer-Assisted Generation of Antimony-Doped SnO2 Nanoparticles with High Crystallinity for Application in Gas Sensors. Small 2008, 10, 1656–1660. [Google Scholar] [CrossRef]
- Murakami, K.; Yagi, I.; Kaneko, S. Oriented Growth of Tin Oxide Thin Films on glass Substrates by Spray Pyrolysis of Organotin Compounds. J. Am. Ceram. Soc. 1996, 79, 2557–2562. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Valanarasu, S.; Jeyadheepan, K.; Kim, H.-S.; Vikraman, D. Evaluation of the physical, optical, and electrical properties of SnO2:F thin films prepared by nebulized spray pyrolysis for optoelectronics. J. Mater. Sci. Mater. Electron. 2018, 29, 3648–3656. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Lyu, X.; Zhang, W. Preparation and investigation of nano-thick FTO/Ag/FTO multilayer transparent electrodes with high figure of merit. Sci. Rep. 2016, 6, 20399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, Q.; Dong, G.; He, Y.; Diao, X. Influence of thickness on the structure, electrical, optical and electrochromic properties of AZO thin films and their inorganic all solid-state devices. Electrochim. Acta 2017, 258, 1336–1347. [Google Scholar] [CrossRef]
- Koo, B.-R.; Kim, K.-H.; Ahn, H.-J. Novel tunneled phosphorus-doped WO3 films achieved using ignited red phosphorus for stable and fast switching electrochromic performances. Nanoscale 2019, 11, 3318–3325. [Google Scholar]
- Wang, C.-K.; Lin, C.-K.; Wu, C.-L.; Brahma, S.; Wang, S.-C.; Huang, J.-L. Characterization of electrochromic tungsten oxide film from electrochemical anodized RF-sputtered tungsten films. Ceram. Int. 2013, 39, 4293–4298. [Google Scholar] [CrossRef]
- Koo, B.-R.; Kim, K.-H.; Ahn, H.-J. Switching electrochromic performance improvement enabled by highly developed mesopores and oxygen vacancy defects of Fe-doped WO3 films. Appl. Surf. Sci. 2018, 453, 238–244. [Google Scholar] [CrossRef]







| Samples | Bare FTO | 60M-FTO | 40M-FTO | 24M-FTO |
|---|---|---|---|---|
| Carrier concentration (cm−3) | 7.82 × 1020 | 1.02 × 1021 | 1.21 × 1021 | 1.14 × 1021 |
| Mobility (cm2/V·S) | 1.28 × 10 | 1.91 × 10 | 2.28 × 10 | 2.18 × 10 |
| Resistivity (Ω·cm) | 4.97 × 10−4 | 4.51 × 10−4 | 4.27 × 10−4 | 4.59 × 10−4 |
| Sheet resistance (Ω/□) | 8.39 ± 0.17 | 8.98 ± 0.13 | 8.03 ± 0.21 | 8.11 ± 0.24 |
| Transmittance (%) | 80.3 | 89.8 | 88.9 | 86.2 |
| Figure of Merit (Ω−1) | 1.30 × 10−2 | 3.80 × 10−2 | 3.84 × 10−2 | 2.80 × 10−2 |
| Samples | Tbleached (%) | Tcolored (%) | Modulation (%) | Switching Speed (Colored) | Switching Speed (Bleached) | Coloration Efficiency (cm2/C) |
|---|---|---|---|---|---|---|
| Bare FTO | 80.1 | 31.3 | 48.8 | 11.0 | 11.0 | 38.2 |
| 60M-FTO | 88.3 | 38.3 | 50.0 | 7.5 | 10.3 | 42.6 |
| 40M-FTO | 87.0 | 28.3 | 58.7 | 4.1 | 5.9 | 52.4 |
| 24M-FTO | 83.3 | 31.9 | 54.5 | 6.2 | 9.5 | 47.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-J.; Kim, K.-H.; Ahn, H.-J. Net-Patterned Fluorine-Doped Tin Oxide to Accelerate the Electrochromic and Photocatalytic Interface Reactions. Catalysts 2021, 11, 249. https://doi.org/10.3390/catal11020249
Jeong S-J, Kim K-H, Ahn H-J. Net-Patterned Fluorine-Doped Tin Oxide to Accelerate the Electrochromic and Photocatalytic Interface Reactions. Catalysts. 2021; 11(2):249. https://doi.org/10.3390/catal11020249
Chicago/Turabian StyleJeong, Seock-Joon, Kue-Ho Kim, and Hyo-Jin Ahn. 2021. "Net-Patterned Fluorine-Doped Tin Oxide to Accelerate the Electrochromic and Photocatalytic Interface Reactions" Catalysts 11, no. 2: 249. https://doi.org/10.3390/catal11020249