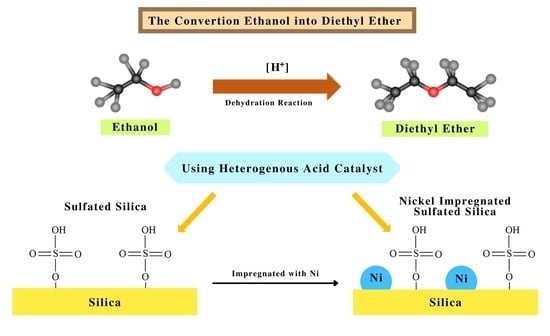
1. Introduction
The world’s demand for energy originating from fossil fuels such as natural gas and petroleum oil continues to increase, while the availability of this fuel continues to decrease. The development of alternative fuels is one solution to reducing this [
1,
2]. Biofuels are environmentally friendly and renewable alternative energy sources to overcome this problem. Diethyl ether is one of the biofuels that can effectively be used as a fuel [
3] due to its high octane and cetane numbers, with values of 110 and 125, respectively [
4,
5,
6,
7]. Its good combustion characteristics indicate that it can improve engine performance and reduce fuel consumption [
8]. Diethyl ether (DEE) is generally made by dehydrating ethanol compounds (Barbet process) with a homogeneous catalyst such as H
2SO
4. The conversion of ethanol into diethyl ether using an H
2SO
4 catalyst reached 23% DEE [
9]. However, the drawback of this process is that it is expensive, corrosive, and the separation of catalyst and products at the end of the reaction is still difficult due to the homogeneous catalyst and DEE liquid product being mixed together. A solid acid catalyst is a heterogeneous catalyst and it was already been reported to overcome this problem in ethanol dehydration [
10].
Silica as a catalyst matrix has high surface area, good chemical and thermal stability, uniform pore size, high adsorption capacity, and good pore framework for free diffusion between substrates [
11,
12]. As an inert material, the addition of sulfate ions (SO
42−) to the silica can improve its catalytic activities by increasing the acidity, surface area, thermal stability, and porosity [
13,
14]. The sulfate ions (SO
42−) act as ligands that donate a lone pair electron through the Oxygen atom and form a coordination bond with the Si⁴⁺ cation [
15]. The addition of sulfate anions to silica (SO
4/SiO
2) will create Brønsted and Lewis acid sites from the sulfate group, which increases its catalytic activity in ethanol dehydration. Feng et al. [
16] reported the catalytic activity of Ti(SO
4)
2/CS (Sulfated titania/activated carbon sphere) with catalyst for methanol dehydration into dimethyl ether (DME). Ti(SO
4)
2/CS catalyst reveal the conversion and selectivity of DME up to 11.7 and 75.8%, respectively. The high conversion of DME is due to the presence of weak acid and redox sites on the Ti(SO
4)
2/CS catalyst, which is suitable for direct oxidation of dimethyl ether.
Increasing the catalytic activity of SiO
2 catalyst has been widely investigated by the addition of metals. Platinum (Pt) and Palladium (Pd) noble metals are commonly used as dopants for SiO
2 supported catalyst fabrication. Supported Pt and Pd metal catalyst shows higher catalytic activity. However, these metals are less economical due to their high cost and limited availability. Pt and Pd metals are starting to be switched with non-noble transition metal that has low cost and is more available, such as Nickel (Ni) [
17]. The catalytic activity of Nickel (Ni) as a support catalyst shows its good activity and acidity. Dehydration of ethanol to diethyl ether using Nickel (Ni) as a support catalyst has shown good catalytic activity and reached a conversion of diethyl ether up to 30% [
18,
19]. Ni metal is a type of transition metal that can act as a carrier in the adsorption of reactants on the surface of the catalyst. Ni metal forms coordination covalent bonds which can facilitate the formation of intermediates on the surface of the catalyst. In addition, Nickel has an unpaired electron which is capable of forming Brønsted acid site by hydrogen (H
2) splitting and a Lewis acid site as an electron acceptor from vacant of orbital (4p) that can accept electron pairs from Lewis bases [
20,
21]. Recent reports reveal that the addition of Nickel to a SiO
2 catalyst supports and enhances the hydrocracking of vegetable oil due to the presence of Ni dopants in the supported catalyst [
21].
This study focused on the development of porous sulphated SiO2 as a catalyst for the dehydration of ethanol into diethyl ether. The influence of sulfation on SiO2 (SO4/SiO2 catalyst) was determined by the acidity value of the optimum sulfate concentration and calcination temperature. The catalyst with the highest acidity value was then impregnated with Nickel metal as Ni-SO4/SiO2. Based on the highest acidity of the catalyst, SO4/SiO2 and Ni-SO4/SiO2 was applied to determine the catalyst efficiency in the dehydration reaction of ethanol into diethyl ether.
3. Experimental
3.1. Materials
The materials used in this study were ethanol (C2H5OH; 96%), tetraethyl orthosilicate (TEOS), methanol (CH3OH), hydrochloric acid (HCl; 37%), sulfuric acid (H2SO4; 98%), pyridine (C5H5N), ammonia (NH3), nickel chloride hexahydrate (NiCl2·6H2O), nitrogen gas, and sodium bicarbonate (NaHCO3; 99%), all of them obtained from Merck.
3.2. SO4/SiO2 Catalyst Preparation
A total of 16.8 mL TEOS, 30 mL ethanol, and 10 mL H2SO4 (with concentrations of 1, 2, 3 M) were mixed and then stirred to form a gel. The gel was heated in an oven for 3 h at 100 °C and refluxed with methanol for 72 h. The solid was separated by centrifugation for 20 min at 2000 rpm and dried at 80 °C. The solids produced were calcined at 500 °C and labeled as SS-1-500, SS-2-500, and SS-3-500, later tested for their acidity. The catalyst with the highest acidity was calcined at temperatures of 400, 500, and 600 °C and then labeled as SS-x-400, SS-x-500, and SS-x-600. Each catalyst was characterized using FTIR and XRD. This was followed, again, with an acidity test. The catalyst with the highest acidity was then characterized using SEM, SAA, and TGA/DSC.
3.3. Ni-SO4/SiO2 Catalyst Preparation
The catalyst with the highest acidity was then impregnated with Nickel metal with concentrations of 1, 2, and 3%. Impregnation was achieved by stirring for 24 h at room temperature. The solid was separated by centrifugation at 2000 rpm and dried in an oven at 100 °C before being calcined at 500 °C for 4 h. The Ni-SS-2 catalysts were labeled as 1%/Ni-SS-2, 2%/Ni-SS-2, and 3%/Ni-SS-2 and tested for their acidity value and characterized using acidity test, FTIR, and AAS.
3.4. Ethanol Dehydration into Diethyl Ether
The catalytic activity of the SiO2, SO4/SiO2 and Ni-SO4/SiO2 catalysts for the dehydration of ethanol to diethyl ether was tested in a reactor furnace system batch equipped with temperature and pressure controllers. A total of 20 mL of ethanol and catalyst (2 wt%) were fed into reactor in a different batch and flowed the N2 gas. The catalytic activity and selectivity were carried out at temperatures of 175, 200, and 225 °C with constant liquid hourly space velocity (LHSV). The DEE product was then characterized using GCMS analysis.
3.5. Characterization
Catalyst characterization was carried out using Fourier Transform InfraRed (FTIR) spectroscopy using Shimadzu Prestige-21 (Tokyo, Japan) with a wave number range of 4000–400 cm−1 and the KBr pellet technique. X-ray Diffraction (XRD) analysis was performed using the X’pert Pro PANalytical (Morgan Hill, CA, USA) with a Cu X-ray tube (1.5406 Å). The acidity test was carried out gravimetrically using pyridine vapor. Scanning Electron Microscope (SEM) imaging and elemental analysis were performed using High-Tech’s scanning electron microscope (Hitachi) SU 3500 (Tokyo, Japan). Surface area analyzer (SAA) was performed using Quantachrome Quadrasorb-Evo Surface Area and Pore (London, UK). Size Analyzer Thermogravimetry analysis and Differential Scanning Calorimeter (TGA/DSC) were carried out in a free atmosphere with a temperature range of 30–1200 °C (temperature increase rate of 10 °C/minute). Atomic Absorption Spectrometer (AAS) was carried out using Perkin Elmer, 5100 PC (Tokyo, Japan); analysis of the liquid products used Gas Chromatography (GC) GC 148 Shimadzu (FID) (Tokyo, Japan) with HP5 chromatography column (5% Phenyl Methyl Siloxane).