Insight into Composition and Intermediate Evolutions of Copper-Based Catalysts during Gas-Phase CO2 Electroreduction to Multicarbon Oxygenates
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
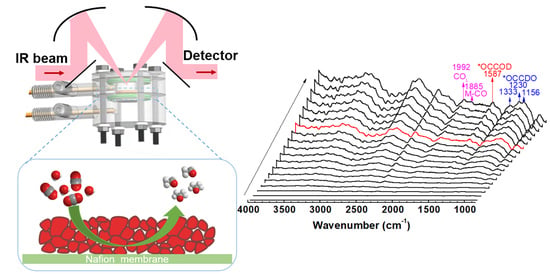
3.3. Catalyst Characterization
3.4. Electrochemical Measurements
3.5. Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS)
3.6. Operando Raman Spectroscopy
3.7. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.C.; Wolfram, C.; de Jong, K.P.; Gross, R.; Lewis, N.S.; Boardman, B.; Ragauskas, A.J.; Ehrhardt-Martinez, K.; Crabtree, G.; Ramana, M.V. The frontiers of energy. Nat. Energy 2016, 1, 15020. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, Z.B.; Gray, T.S.; Xu, Y.; Lin, Q.; Gunnoe, T.B.; Zangari, G. High selectivity towards formate production by electrochemical reduction of carbon dioxide at copper-bismuth dendrites. ChemSusChem 2019, 12, 231–239. [Google Scholar] [CrossRef]
- Reske, R.; Mistry, H.; Behafarid, F.; Roldan Cuenya, B.; Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent. Sci. 2016, 2, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Jung, H.; Kim, N.K.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhu, C.; Wang, K.; Li, G.; Dong, X.; Song, Y.; Xue, J.; Chen, W.; Wei, W.; Sun, Y. Promotion of CO2 electrochemical reduction via Cu nanodendrites. ACS Appl. Mater. Interfaces 2020, 12, 11562–11569. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu hybrid foams as high-performance electrocatalysts for oxygen evolution reaction in alkaline media. ACS Catal. 2016, 7, 986–991. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, X.; Yang, D.; Ma, J.; Kang, X.; Zheng, L.; Zhang, J.; Wu, Z.; Han, B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-C.; Chang, C.-C.; Chiu, S.-Y.; Pai, H.-T.; Liao, T.-Y.; Hsu, C.-S.; Chiang, W.-H.; Tsai, M.-K.; Chen, H.M. Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction. Nat. Commun. 2020, 11, 3525. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.; Scholten, F.; Thanh, T.N.; Sinev, I.; Timoshenko, J.; Wang, X.; Jovanov, Z.; Gliech, M.; Roldan Cuenya, B.; Varela, A.S.; et al. Electrocatalytic CO2 reduction on CuOx nanocubes: Tracking the evolution of chemical state, geometric structure, and catalytic selectivity using operando spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 17974–17983. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.; Lee, J. Electrocatalytic Production of C3-C4 Compounds by Conversion of CO2 on a Chloride-Induced Bi-Phasic Cu2O-Cu Catalyst. Angew. Chem. Int. Ed. 2015, 54, 14701–14705. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, S.; Cheng, D.; Li, L.; Zhu, W.; Zhong, D.; Zhao, Z.J.; Li, J.; Wang, T.; Gong, J. Controllable Cu0 -Cu+ sites for electrocatalytic reduction of carbon dioxide. Angew. Chem. Int. Ed. 2021, 60, 15344–15347. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Guerra, N.; Moreno-López, L.; Serrano-Ruiz, J.C.; Valverde, J.L.; de Lucas-Consuegra, A. Gas phase electrocatalytic conversion of CO2 to syn-fuels on Cu based catalysts-electrodes. Appl. Catal. B 2016, 188, 272–282. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Lai, Z. Synthesis of core–shell heterostructured Cu/Cu2O nanowires monitored by in situ XRD as efficient visible-light photocatalysts. J. Mater. Chem. A 2013, 1, 13862–13868. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: Identification of CuIII oxides as catalytically active species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Deng, Y.; Yeo, B.S. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando Raman spectroscopy. ACS Catal. 2017, 7, 7873–7889. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(I) oxide catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Zhuang, T.T.; Seifitokaldani, A.; Li, J.; Huang, C.W.; Tan, C.S.; Li, Y.; De Luna, P.; Dinh, C.T.; Hu, Y.; et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 2018, 9, 3828. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.J.; Chang, X.; Mu, R.; Zha, S.; Zhang, G.; Gong, J. The functionality of surface hydroxy groups on the selectivity and activity of carbon dioxide reduction over cuprous oxide in aqueous solutions. Angew. Chem. Int. Ed. 2018, 57, 7724–7728. [Google Scholar] [CrossRef]
- Dong, X.; Li, G.; Chen, W.; Zhu, C.; Li, T.; Song, Y.; Sun, N.; Wei, W. Gas-phase CO2 electroreduction over Sn–Cu hollow fibers. Mater. Adv. 2021, 2, 241–247. [Google Scholar] [CrossRef]
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.; Héroguel, F.; Steier, L.; Ahmad, S.; Luterbacher, J.S.; Mayer, M.T.; Luo, J.; Grätzel, M. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2017, 2, 17087. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, Z.; Zhang, X.; Li, L.; Li, Y.; Xu, H.; Li, X.; Yu, X.; Zhang, Z.; Liang, Y.; et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, M.H.; Huang, B. Cu2O nanoparticles with both {100} and {111} facets for enhancing the selectivity and activity of CO2 electroreduction to ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef] [Green Version]
- Perez-Gallent, E.; Figueiredo, M.C.; Calle-Vallejo, F.; Koper, M.T. Spectroscopic observation of a hydrogenated CO dimer intermediate during CO reduction on Cu(100) electrodes. Angew. Chem. Int. Ed. 2017, 56, 3621–3624. [Google Scholar] [CrossRef]
- Schouten, K.J.; Qin, Z.; Perez Gallent, E.; Koper, M.T. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J. Am. Chem. Soc. 2012, 134, 9864–9867. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, J.D.; Bell, A.T.; Head-Gordon, M. Identification of possible pathways for C–C bond formation during electrochemical reduction of CO2: New theoretical insights from an improved electrochemical model. J. Phys. Chem. Lett. 2016, 7, 1471–1477. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhao, Y.; Li, J.P.H.; Chen, W.; Li, S.; Dong, X.; Song, Y.; Yang, Y.; Wei, W.; Sun, Y. Insight into Composition and Intermediate Evolutions of Copper-Based Catalysts during Gas-Phase CO2 Electroreduction to Multicarbon Oxygenates. Catalysts 2021, 11, 1502. https://doi.org/10.3390/catal11121502
Li G, Zhao Y, Li JPH, Chen W, Li S, Dong X, Song Y, Yang Y, Wei W, Sun Y. Insight into Composition and Intermediate Evolutions of Copper-Based Catalysts during Gas-Phase CO2 Electroreduction to Multicarbon Oxygenates. Catalysts. 2021; 11(12):1502. https://doi.org/10.3390/catal11121502
Chicago/Turabian StyleLi, Guihua, Yonghui Zhao, Jerry Pui Ho Li, Wei Chen, Shoujie Li, Xiao Dong, Yanfang Song, Yong Yang, Wei Wei, and Yuhan Sun. 2021. "Insight into Composition and Intermediate Evolutions of Copper-Based Catalysts during Gas-Phase CO2 Electroreduction to Multicarbon Oxygenates" Catalysts 11, no. 12: 1502. https://doi.org/10.3390/catal11121502