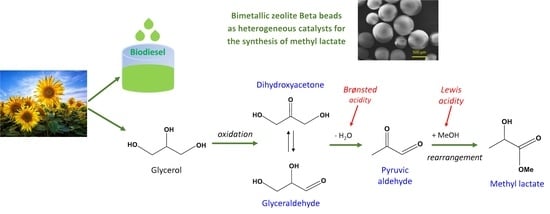
Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Catalyst Synthesis
3.3. Characterization of the Catalysts
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References and Notes
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Biobased chemicals from conception toward industrial reality: Lessons learned and to be learned. ACS Catal. 2012, 2, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Corma Canos, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Tang, Z.; Boer, D.G.; Syariati, A.; Enache, M.; Rudolf, P.; Heeres, H.J.; Pescarmona, P.P. Base-free conversion of glycerol to methyl lactate using a multifunctional catalytic system consisting of Au-Pd nanoparticles on carbon nanotubes and Sn-MCM-41-XS. Green Chem. 2019, 21, 4115–4126. [Google Scholar] [CrossRef] [Green Version]
- De Clippel, F.; Dusselier, M.; Van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and selective sugar conversion to alkyl lactate and lactic acid with bifunctional carbon-silica catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yabushita, M.; Kim, M.; Hirayama, J.; Motokura, K.; Fukuoka, A.; Nakajima, K. Catalytic Conversion of Biomass-Derived Carbohydrates to Methyl Lactate by Acid-Base Bifunctional γ-Al2O3. ACS Sustain. Chem. Eng. 2018, 6, 8113–8117. [Google Scholar] [CrossRef]
- West, R.M.; Holm, M.S.; Saravanamurugan, S.; Xiong, J.; Beversdorf, Z.; Taarning, E.; Christensen, C.H. Zeolite H-USY for the production of lactic acid and methyl lactate from C3-sugars. J. Catal. 2010, 269, 122–130. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Janssen, K.P.F.; Delaet, C.; Stroobants, C.; Houthoofd, K.; Philippaerts, A.; De Jonghe, C.; Paul, J.S.; Jacobs, P.A.; Sels, B.F. Zeolite-catalysed conversion of C3 sugars to alkyl lactates. Green Chem. 2010, 12, 1083–1089. [Google Scholar] [CrossRef]
- Osmundsen, C.M.; Spangsberg Holm, M.; Dahl, S.; Taarning, E. Tin-containing silicates: Structure-activity relations. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2000–2016. [Google Scholar] [CrossRef] [Green Version]
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Fiorilli, S.L.; Heeres, H.J.; Pescarmona, P.P. Multifunctional Heterogeneous Catalysts for the Selective Conversion of Glycerol into Methyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 10923–10933. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Zheng, M.; Li, X.; Song, L.; Sun, R.; Sebastian, J.; Wang, A.; Wang, J.; Wang, X.; Zhang, T. Catalytic Conversion of Carbohydrates to Methyl Lactate Using Isolated Tin Sites in SBA-15. ChemistrySelect 2017, 2, 309–314. [Google Scholar] [CrossRef]
- Jasra, R.V.; Tyagi, B.; Badheka, Y.M.; Choudary, V.N.; Bhat, T.S.G. Effect of clay binder on sorption and catalytic properties of zeolite pellets. Ind. Eng. Chem. Res. 2003, 42, 3263–3272. [Google Scholar] [CrossRef]
- Michels, N.L.; Mitchell, S.; Pérez-Ramírez, J. Effects of binders on the performance of shaped hierarchical MFI zeolites in methanol-to-hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Mitchell, S.; Pérez-Ramírez, J. Hierarchical Zeolites Overcome all Obstacles: Next Stop Industrial Implementation. Chim. Int. J. Chem. 2013, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Li, L.; Sels, B.F.; Jacobs, P.A.; Pescarmona, P.P. Titanosilicate beads as versatile catalysts for the conversion of trioses to lactates and for the epoxidation of alkenes. Catal. Today 2011, 173, 89–94. [Google Scholar] [CrossRef]
- Cheng, W.; Jiang, Y.; Xu, X.; Wang, Y.; Lin, K.; Pescarmona, P.P. Easily recoverable titanosilicate zeolite beads with hierarchical porosity: Preparation and application as oxidation catalysts. J. Catal. 2016, 333, 139–148. [Google Scholar] [CrossRef]
- Li, L.; Cani, D.; Pescarmona, P.P. Metal-containing TUD-1 mesoporous silicates as versatile solid acid catalysts for the conversion of bio-based compounds into valuable chemicals. Inorg. Chim. Acta 2015, 431, 289–296. [Google Scholar] [CrossRef]
- Dijkmans, J.; Dusselier, M.; Gabriëls, D.; Houthoofd, K.; Magusin, P.C.M.M.; Huang, S.; Pontikes, Y.; Trekels, M.; Vantomme, A.; Giebeler, L.; et al. Cooperative catalysis for multistep biomass conversion with Sn/Al beta zeolite. ACS Catal. 2015, 5, 928–940. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Dai, W.; Wu, G.; Guan, N.; Li, L.; Hunger, M. Improved postsynthesis strategy to Sn-beta zeolites as lewis acid catalysts for the ring-opening hydration of epoxides. ACS Catal. 2014, 4, 2801–2810. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.; Wu, H.; Liu, Y.; Jiang, J.G.; Wu, P. Postsynthesis and selective oxidation properties of nanosized Sn-beta zeolite. J. Phys. Chem. C 2011, 115, 3663–3670. [Google Scholar] [CrossRef]
- Wang, J.; Okumura, K.; Jaenicke, S.; Chuah, G.K. Post-synthesized zirconium-containing Beta zeolite in Meerwein-Ponndorf-Verley reduction: Pros and cons. Appl. Catal. A Gen. 2015, 493, 112–120. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Design of Lewis-acid centres in zeolitic matrices for the conversion of renewables. Chem. Soc. Rev. 2015, 44, 7025–7043. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Collard, X.; Bertrand, A.; Sels, B.F.; Pescarmona, P.P.; Aprile, C. Extra-small porous Sn-silicate nanoparticles as catalysts for the synthesis of lactates. J. Catal. 2014, 314, 56–65. [Google Scholar] [CrossRef]
- Dijkmans, J.; Demol, J.; Houthoofd, K.; Huang, S.; Pontikes, Y.; Sels, B. Post-synthesis Snβ: An exploration of synthesis parameters and catalysis. J. Catal. 2015, 330, 545–557. [Google Scholar] [CrossRef]
- Ramanathan, A.; Klomp, D.; Peters, J.A.; Hanefeld, U. Zr-TUD-1: A novel heterogeneous catalyst for the Meerwein-Ponndorf-Verley reaction. J. Mol. Catal. A Chem. 2006, 260, 62–69. [Google Scholar] [CrossRef]
- Iida, T.; Ohara, K.; Román-Leshkov, Y.; Wakihara, T. Zeolites with isolated-framework and oligomeric-extraframework hafnium species characterized with pair distribution function analysis. Phys. Chem. Chem. Phys. 2018, 20, 7914–7919. [Google Scholar] [CrossRef]
- Danumah, C.; Vaudreuil, S.; Bonneviot, L.; Bousmina, M.; Giasson, S.; Kaliaguine, S. Synthesis of macrostructured MCM-48 molecular sieves. Microporous Mesoporous Mater. 2001, 44–45, 241–247. [Google Scholar] [CrossRef]
- Here, we are assuming that only Sn atoms act as Lewis acid sites. Extraframework Al species that might be present as residues of the dealumination process can also display Lewis acid character, but their contribution to the total Lewis acidity of the catalyst is expected to be minor and is thus omitted from this calculation.
- Gunther, W.R.; Michaelis, V.K.; Griffin, R.G.; Roman-Leshkov, Y. Interrogating the lewis acidity of metal sites in beta zeolites with 15N pyridine adsorption coupled with MAS NMR spectroscopy. J. Phys. Chem. C 2016, 120, 28533–28544. [Google Scholar] [CrossRef] [Green Version]
- Taarning, E.; Saravanamurugan, S.; Holm, M.S.; Xiong, J.; West, R.M.; Christensen, C.H. Zeolite-catalyzed isomerization of triose sugars. ChemSusChem 2009, 2, 625–627. [Google Scholar] [CrossRef]
- Emeis, C.A. ChemInform Abstract: Determination of Integrated Molar Extinction Coefficients for IR Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Vivian, A.; Fusaro, L.; Debecker, D.P.; Aprile, C. Mesoporous Methyl-Functionalized Sn-Silicates Generated by the Aerosol Process for the Sustainable Production of Ethyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 14095–14103. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gonçalves, M.; Mandelli, D.; Pescarmona, P.P.; Carvalho, W.A. Solvent-free conversion of glycerol to solketal catalysed by activated carbons functionalised with acid groups. Catal. Sci. Technol. 2014, 4, 2293–2301. [Google Scholar] [CrossRef]
- da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Wadlinger, R.L.; Kerr, G.T.; Rosinski, E.J. Catalytic Composition of a Crystalline Zeolite, Modil Oil Corporation. U.S. Patent 3,308,069, 7 March 1967. [Google Scholar]
- Iglesias, J.; Moreno, J.; Morales, G.; Melero, J.A.; Juárez, P.; López-Granados, M.; Mariscal, R.; Martínez-Salazar, I. Sn-Al-USY for the valorization of glucose to methyl lactate: Switching from hydrolytic to retro-aldol activity by alkaline ion exchange. Green Chem. 2019, 21, 5876–5885. [Google Scholar] [CrossRef]







| Entry | Catalyst | Specific Surface Area (m2/g) a | [HNO3] for the Dealumination (M) | Si/Al Molar Ratio b | Si/MetalLA Molar Ratio c |
|---|---|---|---|---|---|
| 1 | Parent zeolite Beta beads | 530 | - | 10 | - |
| 2 | Sn-deAl-1.8-Beta-B | 442 | 1.8 | 40 | 50 |
| 3 | Sn-deAl-7.2-Beta-B | 432 | 7.2 | 80 | 133 |
| 4 | Zr-deAl-1.8-Beta-B | 355 | 1.8 | 32 | 13 |
| 5 | Hf-deAl-1.8-Beta-B | 339 | 1.8 | 28 | 55 |
| Catalyst | Yield of ML (%) | Yield of PADA (%) | Selectivity (%) | TONmetalLA | ||
|---|---|---|---|---|---|---|
| ML | PADA | |||||
| 1 | Sn-deAl-1.8-Beta-B | 90 | 0.4 | 99 | 1 | 57 |
| 2 | Sn-deAl-7.2-Beta-B | 76 | 6 | 92 | 8 | 120 |
| 3 | Zr-deAl-1.8-Beta-B | 10 | 53 | 16 | 84 | 2 |
| 4 | Hf-deAl-1.8-Beta-B | 9 | 23 | 28 | 72 | 6 |
| Entry | Catalyst | Lewis Acid Sites (µmol/g) | Brønsted Acid Sites (µmol/g) | Lewis/Brønsted Ratio |
|---|---|---|---|---|
| 150 °C | 150 °C | 150 °C | ||
| 1 | Sn-deAl-1.8-Beta-B | 60 | 13 | 5 |
| 2 | Sn-deAl-7.2-Beta-B | 48 | 3 | 16 |
| Entry | Catalyst | Conv. (%) | Yield of ML (%) | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ML | MP | MGe | DMT | MGo | DMO | GF | ||||
| 1 | Sn-deAl-1.8-Beta-B | 29 | 20 | 67 | n.d. | 3.9 | 7.1 | 5.8 | 4.8 | 5.8 |
| 2 | Sn-MCM-41-XS a | 81 | 70 | 87 | 5.6 | 5.1 | 0.3 | 1.6 | 0.2 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgar Pour, Z.; Boer, D.G.; Fang, S.; Tang, Z.; Pescarmona, P.P. Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate. Catalysts 2021, 11, 1346. https://doi.org/10.3390/catal11111346
Asgar Pour Z, Boer DG, Fang S, Tang Z, Pescarmona PP. Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate. Catalysts. 2021; 11(11):1346. https://doi.org/10.3390/catal11111346
Chicago/Turabian StyleAsgar Pour, Zahra, Dina G. Boer, Shun Fang, Zhenchen Tang, and Paolo P. Pescarmona. 2021. "Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate" Catalysts 11, no. 11: 1346. https://doi.org/10.3390/catal11111346