Mesoporous TiO2 Implanted ZnO QDs for the Photodegradation of Tetracycline: Material Design, Structural Characterization and Photodegradation Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation
2.1.1. X-ray Diffraction Analysis
2.1.2. Morphology
2.1.3. X-ray Photoelectron (XPS) Analysis
2.1.4. Optical Absorption Properties
2.1.5. Specific Surface Area and Pore Structure
2.1.6. Photoluminescence Studies
2.2. Photocatalytic Activity
2.2.1. The Effect of Different Types of Photocatalyst
2.2.2. The Effect of the Initial pH of the Solution
2.2.3. The Effect of the Initial Concentration of TC Solution
2.2.4. The Effect of Photocatalyst Dosage
2.2.5. Scavenging Test
2.2.6. Reusability and Mineralisation Studies
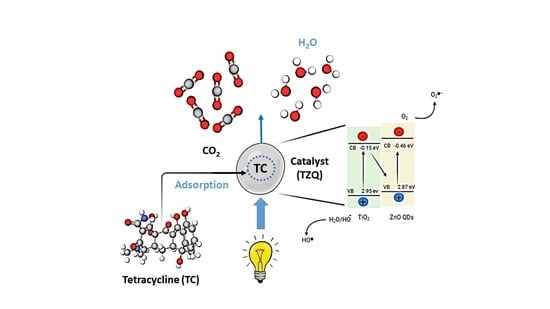
2.2.7. Photocatalytic Degradation Mechanism
2.2.8. Identification of TC Photodegradation Intermediates
2.3. Theoretical Prediction of Tetracycline Degradation Using DFT Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZnO QDs
3.3. Synthesis of Mesoporous TiO2 Implanted ZnO QDs
3.4. Characterisation
3.5. Photodegradation of Tetracycline (TC)
3.6. Analytical Method
3.7. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. A critical review on the recent progress of synthesising techniques and fabrication of TiO2-based nanotubes photocatalysts. Appl. Catal. A Gen. 2014, 481, 127–142. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Colmenares, J.C.; Marinas, A.; Marinas, J.M.; Navío, J.A.; Urbano, F.J. Modification of the photocatalytic activity of Pd/TiO2 and Zn/TiO2 systems through different oxidative and reductive calcination treatments. Appl. Catal. B Environ. 2008, 80, 88–97. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kostov, K.L.; Gegova, R. Sol-gel powder synthesis in the TiO2-TeO2-ZnO system: Structural characterisation and properties. Arab. J. Chem. 2020, 13, 7132–7146. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2018, 160, 154–163. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Scotti, R.; Di Credico, B.; Redaelli, M. Synthesis and Characterisation of Morphology-Controlled TiO2 Nanocrystals. Morphological, Compositional, and Shape Control of Materials for Catalysis. Stud. Surf. Sci. Catal. 2017, 177, 477–540. [Google Scholar]
- Ali, M.M.; Haque, J.; Kabir, M.H.; Kaiyum, M.A.; Rahman, M. Nano synthesis of ZnO–TiO2 composites by sol-gel method and evaluation of their antibacterial, optical and photocatalytic activities. Results Mater. 2021, 11, 100199. [Google Scholar] [CrossRef]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Zalfani, M.; van der Schueren, B.; Mahdouani, M.; Bourguiga, R.; Yu, W.-B.; Wu, M.; Deparis, O.; Li, Y.; Su, B.-L. ZnO quantum dots decorated 3DOM TiO2 nanocomposites: Symbiose of quantum size effects and photonic structure for highly enhanced photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2016, 199, 187–198. [Google Scholar] [CrossRef]
- Patra, M.; Manoth, M.; Singh, V.; Gowd, G.S.; Choudhry, V.; Vadera, S.R.; Kumar, N. Synthesis of stable dispersion of ZnO quantum dots in aqueous medium showing visible emission from bluish green to yellow. J. Lumin. 2009, 129, 320–324. [Google Scholar] [CrossRef]
- Huang, W.; Lv, X.; Tan, J.; Huang, Q.; Cheng, H.; Feng, J.; Li, L. Regulable preparation of the oxygen vacancy of ZnO QDs and their fluorescence performance. J. Mol. Struct. 2019, 1195, 653–658. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, X.J. Synthesis of M@ TiO2 (M= Au, Pd, Pt) core–shell nanocomposites with tunable photo-reactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Mir, N.A.; Qutub, N.; Mehraj, O.; Sabir, S.; Muneer, M. Synthesis, characterisation and optimisation of photocatalytic activity of TiO2/ZrO2 nanocomposite heterostructures. Mater. Sci. Eng. C 2015, 193, 137–145. [Google Scholar] [CrossRef]
- Iqbal, A.; Saidu, U.; Adam, F.; Sreekantan, S.; Yahaya, N.; Ahmad, M.; Ramalingam, R.; Wilson, L.D. Floating ZnO QDs-Modified TiO2/LLDPE Hybrid Polymer Film for the Effective Photodegradation of Tetracycline under Fluorescent Light Irradiation: Synthesis and Characterisation. Molecules 2021, 26, 2509. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Ren, T.Z.; Vantomme, A.; Su, B.L. Facile and generalised preparation of hierarchically mesoporous−macroporous binary metal oxide materials. Chem. Mater. 2004, 16, 5096–5106. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptisation of titanium iso-propoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Lin, M.; Jing, G.; Shen, H.; Yuan, C.S.; Huang, Z.; Wu, X.; Liu, C.W. Mechanism of enhancement of photodegradation of Hg0 by CeO2-TiO2: Effect of band structure on the formation of free radicals. Chem. Eng. J. 2020, 382, 122827. [Google Scholar] [CrossRef]
- Rai, P.; Jo, J.N.; Lee, I.H.; Yu, Y.T. Fabrication of flower-like ZnO microstructures from ZnO nanorods and their photo-luminescence properties. Mater. Chem. Phys. 2010, 124, 406–412. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.S.; Hahn, Y.B. Ammonium ion detection in solution using ver-tically grown ZnO nanorod based field-effect transistor. RSC Adv. 2016, 6, 54836–54840. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, Q.; Zhao, W.; Yu, L.; Qu, H. Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature. Appl. Surf. Sci. 2014, 289, 237–244. [Google Scholar] [CrossRef]
- Xiao-Quan, C.; Huan-Bin, L.; Guo-Bang, G. Preparation of nanometer crystalline TiO2 with high photocatalytic activity by pyrolysis of titanyl organic compounds and photo-catalytic mechanism. Mater. Chem. Phys. 2005, 91, 317–324. [Google Scholar] [CrossRef]
- Rajeena, U.; Akbar, M.; Raveendran, P.; Ramakrishnan, R.M. Graphene reduction of P25 titania: Ti3+-doped titania/graphene nanohybrids for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 26, 9564–9574. [Google Scholar] [CrossRef]
- Mahjoub, M.A.; Monier, G.; Robert-Goumet, C.; Réveret, F.; Echabaane, M.; Chaudanson, D.; Petit, M.; Bideux, L.; Gruzza, B. Synthesis and Study of Stable and Size-Controlled ZnO–SiO2 Quantum Dots: Application as a Humidity Sensor. J. Phys. Chem. C 2016, 120, 11652–11662. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Xu, Q. Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mekasuwandumrong, O.; Chaitaworn, S.; Panpranot, J.; Praserthdam, P. Photocatalytic liquid-phase selective hydrogenation of 3-Nitrostyrene to 3-vinylaniline of various treated-TiO2 without use of reducing gas. Catalysts 2019, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dong, K.; Liu, Z.; Wang, H.; Ma, S.; Zhang, A.; Li, M.; Yu, L.; Li, Y. Sulfurized hematite for photo-Fenton catalysis. Prog. Nat. Sci. 2017, 27, 443–451. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B 2015, 168, 1–8. [Google Scholar]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.(M.); Cheng, H.-M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Hassanien, A.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Rocha, J.; Ananias, D.; Paz, F. Photoluminescent Zeolite-Type Lanthanide Silicates. In Comprehensive Inorganic Chemistry II; Elsevier BV: Amsterdam, The Netherlands, 2013; pp. 87–110. [Google Scholar]
- Reinosa, J.J.; Docio, C.M.Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Suyana, P.; Ganguly, P.; Nair, B.N.; Mohamed, A.P.; Warrier, K.G.K.; Hareesh, U.S. Co3O4–C3N4 p–n nano-heterojunctions for the simultaneous degradation of a mixture of pollutants under solar irradiation. Environ. Sci. Nano 2017, 4, 212–221. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, W.; Lim, Z.S.; You, L.; Wang, J. CdS sensitised 3D hierarchical TiO2/ZnO heterostructure for efficient solar energy conversion. Sci. Rep 2014, 4, 1–6. [Google Scholar]
- Imam, S.S.; Adnan, R.; Kaus, N.H.M. Influence of yttrium doping on the photocatalytic activity of bismuth oxybromide for ciprofloxacin degradation using indoor fluorescent light illumination. Res. Chem. Intermed. 2018, 44, 5357–5376. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Paul, K.K.; Giri, P.K. Role of surface plasmons and hot electrons on the multi-Step photocatalytic decay by detect enriched Ag@TiO2 nanorods under visible light. J. Phys. Chem. C 2017, 121, 20016–20030. [Google Scholar] [CrossRef]
- Khan, H.; Swati, I.K. Fe3+-doped anatase TiO2 with d–d transition, oxygen vacancies and Ti3+ centers: Synthesis, characterisation, uv–vis photocatalytic and mechanistic studies. Ind. Eng. Chem. Res. 2016, 55, 6619–6633. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Evidence of oxygen vacancy induced room temperature ferromagnetism in solvothermally synthesised undoped TiO2 nanoribbons. Nanoscale 2013, 12, 5476–5488. [Google Scholar] [CrossRef]
- Ghorai, A.; Bayan, S.; Gogurla, N.; Midya, A.; Ray, S.K. Highly Luminescent WS2 Quantum Dots/ZnO Heterojunctions for Light Emitting Devices. ACS Appl. Mater. Interfaces 2017, 9, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photo-luminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Vempati, S.; Mitra, J.; Dawson, P. One-step synthesis of ZnO nanosheets: A blue-white fluorophore. Nanoscale Res. Lett. 2012, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A comparative analysis of deep level emission in ZnO layers deposited by various methods. J. Appl Phys. 2009, 105, 1–6. [Google Scholar] [CrossRef]
- Cao, B.; Cai, W.; Zeng, H. Temperature-dependent shifts of three emission bands for ZnO nanoneedle arrays. Appl. Phys. Lett. 2006, 88, 161101. [Google Scholar] [CrossRef]
- Nasseh, N.; Panahi, A.H.; Esmati, M.; Daglioglu, N.; Asadi, A.; Rajati, H.; Khodadoost, F. Enhanced photocatalytic degradation of tetracycline from aqueous solution by a novel magnetically separable FeNi3/SiO2/ZnO nanocomposite under simulated sunlight: Efficiency, stability, and kinetic studies. J. Mol. Liq. 2020, 301, 112434. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Li, Y.; Wang, Y.; Zhang, C.; Zhang, W.; Wang, L.; Niu, L. Enhanced photocatalytic degradation of ciprofloxacin using novel C-dot@Nitrogen deficient g-C3N4: Synergistic effect of nitrogen defects and C-dots. Appl. Surf. Sci. 2019, 465, 450–458. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterisations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, T.; Chen, B.; Malkoske, T.; Yu, S.; Tang, Y. Degradation of Tetracycline with BiFeO3 Prepared by a Simple Hydrothermal Method. Materials 2015, 8, 6360–6378. [Google Scholar] [CrossRef] [PubMed]
- Safari, G.; Hoseini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2014, 12, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Hou, C.; Xie, J.; Yang, H.; Chen, S.; Liu, H. Preparation of Cu2O@TiOF2/TiO2 and its photocatalytic degradation of tetra-cycline hydrochloride wastewater. RSC Adv. 2019, 9, 37911–37918. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Gu, J.; Han, Y.; Gao, Y.; Zong, Y.; Ye, Z.; Xue, J. Facile Fabrication of C–TiO2 Nanocomposites with Enhanced Photocatalytic Activity for Degradation of Tetracycline. ACS Omega 2019, 4, 21063–21071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, P.; Bettini, S.; Sawalha, S.; Pal, S.; Licciulli, A.; Marzo, F.; Lovergine, N.; Valli, L.; Giancane, G. Photocatalytic Degradation of Tetracycline by ZnO/γ-Fe2O3 Paramagnetic Nanocomposite Material. Nanomaterials 2020, 10, 1458. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Lakshminarayana, B.; Suryakala, D.; Subrahmanyam, C. Reduced graphene oxide supported ZnO quantum dots for visible light-induced simultaneous removal of tetracycline and hexavalent chromium. RSC Adv. 2020, 10, 20494–20503. [Google Scholar] [CrossRef]
- Mohamed, R.; Ismail, A.A.; Kadi, M.W.; Alresheedi, A.S.; Mkhalid, I.A. Facile Synthesis of Mesoporous Ag2O−ZnO Hetero-junctions for Efficient Promotion of Visible Light Photodegradation of Tetracycline. ACS Omega 2020, 5, 33269–33279. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: Graphene oxide/magnetite/cerium-doped titania. J. Colloid Interface Sci. 2016, 467, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Thi, V.H.T.; Lee, B.-K. Great improvement on tetracycline removal using ZnO rod-activated carbon fiber composite prepared with a facile microwave method. J. Hazard. Mater. 2017, 324, 329–339. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Muhammad, Y.; Zhang, H.; Tong, Z.; Yu, B.; Sahibzada, M. Effects of divalent copper on tetracycline degradation and the proposed transformation pathway. Environ. Sci. Pollut. Res. 2019, 27, 5155–5167. [Google Scholar] [CrossRef]
- Olivera, S.; Hu, C.; Nagananda, G.S.; Reddy, N.; Venkatesh, K.; Muralidhara, H.B.; Asiri, A.M. The adsorptive removal of Cr(VI) ions and antibacterial activity studies on hydrothermally synthesised iron oxide and zinc oxide nanocomposite. J Taiwan Inst. Chem. Eng. 2018, 93, 342–349. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittmann, B.E. Enhancing degradation and mineralisation of tetracycline using intimately coupled photocatalysis and biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Ning, R.; Yan, Z.; Luo, J.; Du, B.; Zhan, J.; Liu, L.; Zhang, J. Double photoelectron-transfer mechanism in Ag−AgCl/WO3/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for trimethoprim degradation. J. Hazard. Mater. 2021, 403, 123964. [Google Scholar] [CrossRef] [PubMed]
- Bekbolet, M.; Çınar, Z.; Kılıç, M.; Uyguner, C.S.; Minero, C.; Pelizzetti, E. Photocatalytic oxidation of dinitronaphthalenes: Theory and experiment. Chemosphere 2009, 75, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Wahab, H.S.; Koutselos, A.D. A computational study on the adsorption and OH initiated photochemical and photo-catalytic primary oxidation of aniline. Chem. Phys. 2009, 358, 171–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Shen, J.; Pan, Z.; Guo, Y.; Wong, P.K.; Yu, H. Synthesis of 1D BiI2O17ClxBr2-x nanotube solid solu-tions with rich oxygen vacancies for highly efficient removal of organic pollutants under visible light. Appl. Catal. B 2009, 269, 1–16. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Kaus, N.H.M.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef] [Green Version]










| Samples | XPS (wt.%) | |||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | Si 2p | K 2p | Ti 2p | Zn 2p3 | |
| meso-TiO2 | 3.17 | 42.80 | - | - | 54.02 | - |
| TZQ | 1.33 | 41.50 | 2.71 | 3.69 | 45.65 | 5.12 |
| Samples | Eg (eV) | VB Edge (eV) | CB Edge (eV) |
|---|---|---|---|
| ZnO QDs | 3.50 | 2.87 | −0.46 |
| meso-TiO2 | 3.10 | 2.95 | −0.15 |
| TZQ | 3.33 | 3.07 | −0.26 |
| Catalyst | BET Surface Area (m2g−1) | Pore Size (Å) | Pore Volume (cm3g−1) |
|---|---|---|---|
| meso-TiO2 | 44.0 | 74.9 | 0.165 |
| TZQ | 89.3 | 34.4 | 0.182 |
| Catalyst System | Degradation (%) | k (min−1) | R2 |
|---|---|---|---|
| P25 | 19.9 | 3.21 × 10−3 | 0.967 |
| meso-TiO2 | 35.0 | 6.91 × 10−3 | 0.994 |
| ZnO QDs | 61.8 | 11.24 × 10−3 | 0.993 |
| TZQ | 82.2 | 20.52 × 10−3 | 0.999 |
| Photocatalyst | Dosage (g L−1) | [TC] (mg L−1) | Light Source | Results | Ref. |
|---|---|---|---|---|---|
| N doped TiO2 | 0.2 | 10 | 300 W xenon lamp visible light (420 nm) | 87.0% removal after 120 min, 46.8% mineralization | [50] |
| Cu2O@TiOF2/TiO2 | 0.3 | 10 | 500 W Xe lamp | 96.83% removal after 180 min | [51] |
| C doped TiO2 | 0.2 | 10 | visible light | 90.8% removal after 160 min | [52] |
| ZnO/γ–Fe2O3 | 0.05 | 30 | stimulated solar light using a Solar S class A halogen lamp (100 mW cm−2 light intensity) | 88.52% removal after 150 min | [53] |
| ZnO QDs/reduced graphene oxide | 0.05 | 20 | 24 V, 250 W halogen lamps | 68% removal after 120 min, 70% mineralization. | [54] |
| Ag2O/ZnO | 1.8 | 20 | 300 W Xenon lamp with air as oxygen source | 100% after 120 min. | [55] |
| TZQ | 0.25 | 40 | 48 W compact fluorescent lamp | 94.6% after 90 min of irradiation, 79% TOC removal. | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Saidu, U.; Sreekantan, S.; Ahmad, M.N.; Rashid, M.; Ahmed, N.M.; Danial, W.H.; Wilson, L.D. Mesoporous TiO2 Implanted ZnO QDs for the Photodegradation of Tetracycline: Material Design, Structural Characterization and Photodegradation Mechanism. Catalysts 2021, 11, 1205. https://doi.org/10.3390/catal11101205
Iqbal A, Saidu U, Sreekantan S, Ahmad MN, Rashid M, Ahmed NM, Danial WH, Wilson LD. Mesoporous TiO2 Implanted ZnO QDs for the Photodegradation of Tetracycline: Material Design, Structural Characterization and Photodegradation Mechanism. Catalysts. 2021; 11(10):1205. https://doi.org/10.3390/catal11101205
Chicago/Turabian StyleIqbal, Anwar, Usman Saidu, Srimala Sreekantan, Mohammad Norazmi Ahmad, Marzaini Rashid, Naser M. Ahmed, Wan Hazman Danial, and Lee D. Wilson. 2021. "Mesoporous TiO2 Implanted ZnO QDs for the Photodegradation of Tetracycline: Material Design, Structural Characterization and Photodegradation Mechanism" Catalysts 11, no. 10: 1205. https://doi.org/10.3390/catal11101205