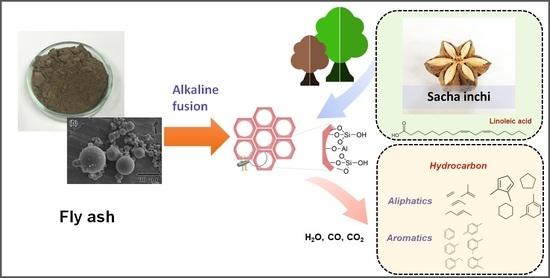
Hydrocarbon Production from Catalytic Pyrolysis-GC/MS of Sacha Inchi Residues Using SBA-15 Derived from Coal Fly Ash
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of SBA-15 Derived from CFA
2.2. Catalytic Pyrolysis of Sacha Inchi Residues
2.2.1. Non-Catalytic Pyrolysis
2.2.2. Effect of Biomass: Catalyst Weight Ratio
2.2.3. Hydrocarbon Distribution and Selectivity
3. Materials and Methods
3.1. Sacha Inchi (SI) Residues
3.2. Synthesis SBA-15 Catalysts
3.2.1. SBA-15 from the Chemical Reagent
3.2.2. SBA-15 Derived from Coal Fly Ash
Acid Treatment of Fly Ash
3.3. Raw Material and Catalyst Characterizations
3.4. Catalyst Activity (Pyrolyzer-GC/MS System)
3.5. Coke Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Nabi, M.D.N. The Potential of Utilising Papaya Seed Oil and Stone Fruit Kernel Oil as Non-Edible Feedstock for Biodiesel Production in Australia—A Review. Energy Rep. 2019, 5, 280–297. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.; Shitao, Y.; Liu, S.; Hailong, Y.; Qiong, W.; Ragauskas, A.J. Catalytic Conversion of Waste Cooking Oils for the Production of Liquid Hydrocarbon Biofuels Using In-Situ Coating Metal Oxide on SBA-15 as Heterogeneous Catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 137–144. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Murdayanti, D.; Ketnawa, S.; Phongthai, S. Chemical Properties and Nutritional Factors of Pressed-Cake from Tea and Sacha Inchi Seeds. Food Biosci. 2016, 15, 64–71. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative Stability and Cold Flow Behavior of Palm, Sacha-Inchi, Jatropha and Castor Oil Biodiesel Blends. Fuel Process. Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Hernando, H.; Fermoso, J.; Ochoa-Hernández, C.; Opanasenko, M.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Performance of MCM-22 Zeolite for the Catalytic Fast-Pyrolysis of Acid-Washed Wheat Straw. Catal. Today 2018, 304, 30–38. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Effect of CV-ZSM-5, Ni-ZSM-5 and FA-ZSM-5 Catalysts for Selective Aromatic Formation from Pyrolytic Vapors of Rubber Wastes. J. Anal. Appl. Pyrolysis 2017, 124, 733–741. [Google Scholar] [CrossRef]
- Mochizuki, T.; Chen, S.Y.; Toba, M.; Yoshimura, Y. Pyrolyzer-GC/MS System-Based Analysis of the Effects of Zeolite Catalysts on the Fast Pyrolysis of Jatropha Husk. Appl. Catal. A Gen. 2013, 456, 174–181. [Google Scholar] [CrossRef]
- Vichaphund, S.; Sricharoenchaikul, V.; Atong, D. Utilization of Fly Ash-Derived HZSM-5: Catalytic Pyrolysis of Jatropha Wastes in a Fixed-Bed Reactor. Environ. Technol. 2017, 38, 1660–1672. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic Pyrolysis of Biomass Components over Mesoporous Catalysts Using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Wen-Zhi, L.; Dong, Z.; Xi-Feng, Z. Analytical Pyrolysis–Gas Chromatography/Mass Spectrometry (Py–GC/MS) of Sawdust with Al/SBA-15 Catalysts. J. Anal. Appl. Pyrolysis 2009, 84, 131–138. [Google Scholar] [CrossRef]
- Kim, S.; Han, Y.; Park, J.; Park, J. Adsorption Characteristics of Mesoporous Silica SBA-15 Synthesized from Mine Tailing. Int. J. Miner. Process. 2015, 140, 88–94. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, W.; Chou, J.; Cai, Z.; Jin, W.; Chen, J.; Xiong, Z.; Ru, X.; Xia, Q. Hydrothermal Synthesis of Plugged Micro/Mesoporous Al-SBA-15 from Spent Fluid Catalytic Cracking Catalyst. Mater. Chem. Phys. 2019, 222, 227–229. [Google Scholar] [CrossRef]
- Chong, C.C.; Abdullah, N.; Bukhari, S.N.; Ainirazali, N.; Teh, L.P.; Setiabudi, H.D. Hydrogen Production via CO2 Reforming of CH4 over Low-Cost Ni/SBA-15 from Silica-Rich Palm Oil Fuel Ash (POFA) Waste. Int. J. Hydrog-Energy 2019, 44, 20815–20825. [Google Scholar] [CrossRef]
- Chandrasekar, G.; You, K.S.; Ahn, J.W.; Ahn, W.S. Synthesis of Hexagonal and Cubic Mesoporous Silica using Power Plant Bottom Ash. Microporous Mesoporous Mater. 2008, 111, 455–462. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous Materials Prepared using Coal Fly Ash as the Silicon and Aluminum Source. J. Mater. Chem. 2001, 11, 3285–3290. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.Q.; Han, Y. Adsorption of Lead Ion on Amino-Functionalized Fly-Ash-Based SBA-15 Mesoporous Molecular Sieves Prepared via a Two-Step Hydrothermal Method. Microporous Mesoporous Mater. 2017, 252, 105–115. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Wang, H.; Ma, J.; Xu, W.Q.; Li, Y.; Han, Y.; Sun, Q. Fe and/or Mn oxides supported on fly ash-derived SBA-15 for low-temperature NH3-SCR. Catal. Commun. 2018, 108, 82–87. [Google Scholar] [CrossRef]
- Ściubidło, A.; Majchrzak-Kucęba, I. Exhaust Gas Purification Process using Fly Ash-Based Sorbents. Fuel 2019, 258, 116126. [Google Scholar] [CrossRef]
- Liang, C.; Wei, M.C.; Tseng, H.H.; Shu, E.C. Synthesis and characterization of the acidic properties and pore texture of Al-SBA-15 supports for the canola oil transesterification. Chem. Eng. J. 2013, 223, 785–794. [Google Scholar] [CrossRef]
- Qoniah, I.; Prasetyoko, D.; Bahruji, H.; Triwahyono, S.; Jalil, A.A.; Purbaningtias, T.E. Direct synthesis of mesoporous aluminosilicates from Indonesian kaolin clay without calcination. Appl. Clay Sci. 2015, 118, 290–294. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Cheng, C.K.; Ng, K.H.; Witoon, T.; Juan, J.C. Ethylene Production from Ethanol Dehydration over Mesoporous SBA-15 Catalyst Derived from Palm Oil Clinker Waste. J. Clean. Prod. 2020, 249, 119323. [Google Scholar] [CrossRef]
- Akti, F. Effect of kaolin on aluminum loading success in synthesis of Al-SBA-15 catalysts: Activity test in ethanol dehydration reaction. Microporous Mesoporous Mater. 2020, 294, 109894. [Google Scholar] [CrossRef]
- Vichaphund, S.; Wimuktiwan, P.; Sricharoenchaikul, V.; Atong, D. In Situ Catalytic Pyrolysis of Jatropha Wastes using ZSM-5 from Hydrothermal Alkaline Fusion of Fly Ash. J. Anal. Appl. Pyrolysis 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha Inchi (Plukenetia volubilis L.): Nutritional Composition, Biological Activity, and Uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- Vicente, J.; De Carvalho, M.G.; Garcia-Rojas, E.E. Fatty Acids Profile of Sacha Inchi Oil and Blends by 1H NMR and GC-FID. Food Chem. 2015, 181, 215–221. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of Jet Fuel and Green Diesel Range Biohydrocarbons by Hydroprocessing of Soybean Oil over Niobium Phosphate Catalyst. Fuel 2019, 245, 446–458. [Google Scholar] [CrossRef]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Pyrolysis of Polyunsaturated Fatty Acids. Fuel Process Technol. 2014, 120, 89–95. [Google Scholar] [CrossRef]
- Kubátová, A.; Ŝávová, J.; Seames, W.S.; Luo, Y.; Sadrameli, S.M.; Linnen, M.J.; Baglayeva, G.V.; Smoliakova, I.P.; Kozliak, E.I. Triacylglyceride Thermal Cracking: Pathways to Cyclic Hydrocarbons. Energy Fuels 2012, 26, 672–685. [Google Scholar] [CrossRef]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Pyrolysis of Millettia (Pongamia) Pinnata Waste for Bio-Oil Production using a Fly Ash Derived ZSM-5 catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 239–249. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.; Wu, L.; Pan, X.; Liang, J.; Sun, Y. Catalytic Fast Pyrolysis of Maize Straw with a Core-Shell ZSM-5@SBA-15 Catalyst for Producing Phenols and Hydrocarbons. Bioresour. Technol. 2019, 289, 121691. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.H.K.; Yusup, S.; Quitain, A.T.; Abdullah, B.; Ameen, M.; Sasaki, M.; Kida, T.; Cheah, K.W. Biogasoline Production from Linoleic Acid via Catalytic Cracking over Nickel and Copper-Doped ZSM-5 Catalysts. Environ. Res. 2020, 186, 109616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Liu, X.; Zhu, S.; Hu, L.; Zhang, Q. Upgrading of Bio-Oil from Catalytic Pyrolysis of Pretreated Rice Husk over Fe-Modified ZSM-5 Zeolite Catalyst. Fuel Process. Technol. 2018, 175, 17–25. [Google Scholar] [CrossRef]
- Ozbay, N.; Yargic, A.S.; Yarbay Sahin, Z.; Yaman, E. Valorization of Banana Peel Waste via In-Situ Catalytic Pyrolysis using Al-Modified SBA-15. Renew. Energy 2019, 140, 633–646. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic Fast Pyrolysis of Biomass over Zeolites for High Quality Bio-Oil—A Review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Kamaruzaman, M.F.; Taufiq-Yap, Y.H.; Derawi, D. Green Diesel Production from Palm Fatty Acid Distillate over SBA-15-Supported Nickel, Cobalt, and Nickel/Cobalt Catalysts. Biomass Bioenergy 2020, 134, 105476. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Soongprasit, K.; Vichaphund, S.; Sricharoenchaikul, V.; Atong, D. Activity of Fly Ash-Derived ZSM-5 and Zeolite X on Fast Pyrolysis of Millettia (Pongamia) Pinnata Waste. Waste Biomass Valoriz. 2020, 11, 715–724. [Google Scholar] [CrossRef]
- Engtrakul, C.; Mukarakate, C.; Starace, A.K.; Magrini, K.A.; Rogers, A.K.; Yung, M.M. Effect of ZSM-5 acidity on aromatic product selectivity during upgrading of pine pyrolysis vapors. Catal. Today 2016, 269, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447–448, 115–123. [Google Scholar] [CrossRef]














| References | Raw Materials | Methods | Zeolites | Applications |
|---|---|---|---|---|
| Kim et al. [12] | Mine tailing | Alkali fusion and hydrothermal treatment | SBA-15 | Cu, Cd, and Pb adsorption |
| Yang et al. [13] | Spent fluid catalytic catalyst | Alkali fusion and hydrothermal treatment | Al/SBA-15 | Catalysis and adsorption |
| Chong et al. [14] | Palm oil fuel ash | Alkali hydrothermal | Ni/SBA-15 | CO2 reforming for H2 production |
| Kumar et al. [16] | Coal fly ash | Alkali fusion | MCM-41, SBA-15 | Catalyst |
| Chandrasekar et al. [15] | Power plant bottom ash | Alkali fusion and hydrothermal treatment | MCM-41, SBA-15, SBA-16 | CO2 capture |
| Li et al. [17] | Coal fly ash | Alkali hydrothermal | SBA-15, NH2-SBA-15 | Lead adsorption |
| Li et al. [18] | Coal fly ash | Alkali hydrothermal | Fe/Mn/Fe-Mn/SBA-15 | NOx removal |
| Ściubidło, A., Majchrzak-Kucęba [19] | Coal fly ash | Alkali fusion and hydrothermal treatment | Na-X, SBA-15, MCM-41 | NO2 capture |
| Sample | Si/Al Ratio | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size c (nm) | Wall Thickness d (nm) |
|---|---|---|---|---|---|
| Fly ash | 1.46 a | 1.28 | - | - | - |
| SBA-15-FA | 144.9 b | 702 | 1.15 | 7.42 | 4.03 |
| SBA-15-Chemical | - | 685 | 1.10 | 6.27 | 4.25 |
| SBA15-Commercial | - | 624 | 1.02 | 5.81 | 5.62 |
| SI:SBA-15 Ratio | Catalysts | Coke (%) |
|---|---|---|
| 1:5 | SBA-15-FA | 6.10 |
| SBA-15-chem | 5.38 | |
| SBA-15-com | 6.17 | |
| 1:10 | SBA-15-FA | 9.27 |
| SBA-15-chem | 7.27 | |
| SBA-15-com | 8.33 |
| Analysis | SI Residues |
|---|---|
| Element, wt % | |
| Carbon | 41.96 |
| Hydrogen | 6.97 |
| Nitrogen | 1.76 |
| Oxygen * | 49.31 |
| Proximate, wt % | |
| Volatiles | 71.63 |
| Fixed carbon | 14.58 |
| Ash | 8.16 |
| Moisture | 5.63 |
| High heating value (HHV, MJ/kg) | 24.9 |
| SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | K2O | MgO | Na2O | TiO2 | P2O5 | MnO | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| 28.55 | 16.06 | 23.4 | 17.03 | 7.37 | 2.17 | 2.44 | 1.72 | 0.48 | 0.25 | 0.14 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soongprasit, C.; Aht-Ong, D.; Sricharoenchaikul, V.; Vichaphund, S.; Atong, D. Hydrocarbon Production from Catalytic Pyrolysis-GC/MS of Sacha Inchi Residues Using SBA-15 Derived from Coal Fly Ash. Catalysts 2020, 10, 1031. https://doi.org/10.3390/catal10091031
Soongprasit C, Aht-Ong D, Sricharoenchaikul V, Vichaphund S, Atong D. Hydrocarbon Production from Catalytic Pyrolysis-GC/MS of Sacha Inchi Residues Using SBA-15 Derived from Coal Fly Ash. Catalysts. 2020; 10(9):1031. https://doi.org/10.3390/catal10091031
Chicago/Turabian StyleSoongprasit, Chakrit, Duangdao Aht-Ong, Viboon Sricharoenchaikul, Supawan Vichaphund, and Duangduen Atong. 2020. "Hydrocarbon Production from Catalytic Pyrolysis-GC/MS of Sacha Inchi Residues Using SBA-15 Derived from Coal Fly Ash" Catalysts 10, no. 9: 1031. https://doi.org/10.3390/catal10091031