Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review
Abstract
:1. Introduction
Carbon Capture and Storage
2. Chemical Looping Processes
2.1. General Introduction
2.2. The Shift in Focus of Chemical Looping Processes
2.3. Reactor Concepts for Chemical Looping
2.3.1. Interconnected Fluidized Bed Systems
2.3.2. Packed-Bed Systems
2.3.3. Rotating Reactor Systems
2.4. Chemical Looping Processes with Focus on Combustion—Energy Production
2.4.1. Syngas-CLC
2.4.2. In Situ Gasification CLC (iG-CLC)
- Initially, the coal is thermally devolatized with rapid pyrolysis and converted into volatile matter, char, and ashes (Equation (6)).
- Subsequently, the remaining char is transformed into syngas in a gasification reaction with the introduced steam or CO2 (respective Equations (7) and (8)), while the water gas shift (Equation (9)) might influence the composition of the final gas mixture.
- Finally, the products from coal gasification as well as the volatile matter from the first reaction react with the solid oxygen carrier, and CO2 and H2O are formed (Equations (10) and (11)).
2.4.3. Chemical Looping with Oxygen Uncoupling (CLOU)
2.4.4. Other CLC Processes
2.5. Chemical Looping with Focus on Chemicals Production
2.5.1. Steam Reforming-Chemical Looping Reforming (SR-CLR)
2.5.2. Autothermal-Chemical Looping Reforming
2.5.3. Chemical Looping Hydrogen Generation
2.5.4. Other Chemical Looping Processes for Chemicals Production
2.6. Chemical Looping Air Separation
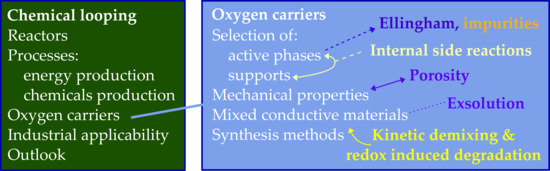
3. Oxygen Carrier Materials
3.1. Oxygen Carrier Properties
- Sufficient oxygen transport capacity (depending on solid inventory in the fuel reactor), considering intra-particle diffusion resistances under operation,
- Favorable thermodynamics regarding fuel and oxidizing agent conversion (depending on the focus of CL-process),
- Favorable selectivity to desired products:
- ◦
- Favorable kinetics regarding fuel and oxidizing agent conversion (depending on residence times in respective reactors),
- ◦
- Negligible carbon deposition, which limits selectivity in most CL-processes,
- Long-term stability (>5000–10,000 h on stream for engineered materials, >2000 h for ores):
- ◦
- Sufficient mechanical properties:
- ▪
- Resistance to attrition for minimizing losses of oxygen carriers during (fluidized bed) operation (<5% AJI (Air Jet Index) according to ASTM D5757-00),
- ▪
- Resistance to fragmentation (crushing strength) (>1 N is a generally accepted lower limit in literature for particles in the range of 100–200 μm).
- ◦
- Negligible agglomeration during process conditions,
- ◦
- Limited sensitivity to impurities in process streams (e.g., H2S),
- Limited cost (raw materials and synthesis) (depending on the economic viability of process),
- Environmentally friendly characteristics, and
- Negligible toxicity.
3.2. Selection of Suitable Active Phases
3.2.1. Thermodynamic Limitations
3.2.2. Oxygen Transfer Capacity
3.2.3. Susceptibility to Impurities
- Ca-containing Mn-based perovskites with the formation of CaSO4 and CaS [67].
3.3. Selection of Suitable Supports
3.3.1. Generally Used Supports
3.3.2. Supports and Their Side-Reactions with the Active Phase
- Mn-based OCs have also shown decreased reactivity over time, which has been attributed to unreactive Mn-silicate formation [166,170]. However, it has also been pointed out that certain Mn-silicates have displayed CLOU properties [93] (due to phase transformations between Mn7SiO12 and MnSiO3 when free SiO2 is present [171]). Mn-based oxygen carriers with large amounts of SiO2 have been reported to deactivate and agglomerate due to sintering during experiments [172]. Besides, a clear loss in oxygen uncoupling ability and reactivity has been observed for materials with higher fractions of SiO2 due to a change in phase composition of the oxygen carriers [171], and they possess low attrition resistance [173].
- TiO2 supports, combined with Fe-based active materials, form FeTiO3 [113]. Unlike the Fe-silicate, this material can be oxidized back by air. They also show a good selectivity towards total oxidation products and are thus suitable for CLC. When ilmenite (Fe-titanate) is used, some studies have pointed out that phase segregation occurs after long-term operation. Pseudobrookite (Fe2TiO5), formed during ilmenite oxidation, segregates into Fe2O3 and TiO2 [174].
- TiO2 supports are usually not used for Mn-based oxygen carriers due to lower reaction rates, although CLOU behavior still remains [179].
- For Cu-based OCs, the formation of CuTiO4 has been reported, which had lower reactivity in the CLC process [157].
- When utilized for Cu-based oxygen carriers, deactivation is observed. The material has high reduction and oxidation rates; however, full conversion does not reach due to the formation of Cu2MgO3, which reduces the attainable oxygen transport capacity of the material [183].
- For Fe-based active phases, the formation of FeMg2O4 is expected. However, this material has not been tested as far as the authors are aware.
- They have shown to react with iron-based active phases with the formation of FeAl2O4 [113,191,192]. This material is regenerable by air [193,194] but is not oxidized back by steam or CO2 [192,195,196], which might result in problems for certain chemical looping applications, unless an excess of active phase is used and maintained in the oxygen carrier.
- When utilized with Ni-based active phases, the formation of NiAl2O4 is reported [197,198,199]. This formation has resulted in lower reaction rates with gaseous fuels compared to NiO, leading to elevated solids inventories necessary for full conversion [200]. Therefore, in general, the use of an excess of NiO is also deemed necessary [24].
- The use of Al2O3-supported Cu-based oxygen carriers has enabled its high-temperature resistance to agglomeration during oxidation [139]; however, long-term experiments have shown slight deactivation phenomena, as the reactivity decreases over time [44,201]. The slower conversions have been attributed to CuAl2O4 and CuAlO2 formation when using Al2O3 supports [183,202,203]. The kinetics for CuAlO2 oxidation is slower compared to the pure Cu-oxides [183,204]. These slow kinetics have resulted in a not fully reversible CuAl2O4 decomposition, which has also a negative influence on the actual oxygen transfer capacity for the oxygen carrier in the process [205,206]. The reactivity of these phases is, however, still very high [41] compared to the other transition metal oxides used as active phases in CL-applications.
- From 2003 onwards, MgAl2O4 supports are already selected several times for Ni-based OC for their improved resistance against sintering and agglomeration during fluidized bed operation [210,211,212]. When employing spray-dried MgAl2O4-supported OC’s, long-term operation is achieved in CLC, and the OC shows an improved performance compared to spray-dried Al2O3-supported OC [213], even at high temperatures due to the chemical stability of MgAl2O4, which inhibits the formation of NiAl2O4 [211,214]. This support stabilizes NiII in the cubic (NiO) phase [215] for multiple cycles [210,216] and improves their regenerability [210,216], even at a temperature above 1300 °C [25]. Moreover, the MgAl2O4-supported OCs display several other advantages when compared to Al2O3-supported OCs, such as higher methane conversion, because of more active phase at the surface of the oxygen carrier [217], a higher selectivity to reforming [213], and a lower tendency for carbon formation [218]. On the other hand, it has also been noted that higher sintering temperatures are necessary for obtaining mechanical properties similar to Al2O3-supported oxygen carriers, while the final porosity of the material is still decreased, indicating a lower mechanical performance [219,220]. Their attrition resistance, which is a better measure for their mechanical performance in the fluidized bed reactors of the process, is, however, shown to be sufficient [211,219].
- When using MgAl2O4-supported Cu-based oxygen carriers, no deactivation has been observed as the reaction between support and active phase to CuAl2O4 or CuAlO2 is avoided [183]. Additionally, problems with agglomeration, sometimes observed when testing Cu-based oxygen carriers supported on Al2O3, have been prevented by using the MgAl2O4 support [204], even up to 1000 °C [183] and in oxygen carriers containing up to 80 wt% CuO [183]. They have also displayed sufficient stability and attrition resistance [183].
- When using these supports for Fe-based oxygen carriers, the formation of FeAl2O4 has been inhibited for at least 20 cycles [25,41,221]. This has resulted in an improved reactivity with both methane [159,222] and gasification products (CO and H2) [223,224,225]. The oxygen carrier is more thermally stable [224] compared to the Al2O3-supported oxygen carrier material, resulting in an improved agglomeration resistance [223]. Still, a series of complex interactions seems to occur, in which Fe ions are taken up into and released from the spinel support, potentially influencing their properties during long-term operation [191,192,226].
- Combinations of Mn–Fe–Si oxides, which display CLOU properties [238],
- CaMnO3 perovskites can be produced from cheap materials (CaO and Mn-ores). They are very promising for CLC as they possess CLOU; however, they are deactivated by sulfur components from the fuel [239]. Sometimes, Ti and Mg are added [180], for achieving higher resistance to sulfur poisoning [151] and better reactivity with the gas gaseous fuels [240]. Ti additionally seems to increase fluidization behavior and perovskite stability [241],
- LaNiO3-supported Fe and Fe–Ce oxygen carriers for hydrogen production. The active phase transforms into CeFeO3, which possesses large amounts of lattice oxygen at the surface [242].
- Cu-based oxygen carriers supported on SrZrO3 and CaZrO3, which possess good CLOU properties and display reduced agglomeration problems [243].
3.4. Mechanical Properties
3.4.1. Crushing Strength
- Crushing strength measurements of an appropriate number of oxygen carrier particles,
- Particle size (range),
- Porosity of the material,
- Composition of the material after synthesis and post-treatment,
- If possible, reaction rates under standardized conditions, relevant for the chemical looping application.
3.4.2. Attrition Resistance
3.5. New Trends in Oxygen Carrier Research and Development
3.5.1. Ionic Conductive and Mixed Conductive Materials
3.5.2. Kinetic Demixing
3.5.3. Exsolution
3.5.4. Stability and Performance of Specific Perovskite and Fluorite Type Materials
3.5.5. Catalyst-Assisted Chemical Looping
3.6. Overview of Synthesis Methods
- Methods starting from solid raw materials (metal oxides or ores), usually with little control on the nanoscale (e.g., by spray-drying),
- Methods starting from metal salts, usually with increased levels of control on the nanoscale (e.g., coprecipitation),
- Activation methods, by which a component, such as an active phase, can be added onto a suitable support material (e.g., impregnation).
3.6.1. Usage of Naturally Occurring Ores and Mechanical Mixing
3.6.2. Freeze Granulation
3.6.3. Spray-Drying
- Homogeneous microstructure
- Scalable process
- Relatively low cost compared to more advanced techniques
- Free-flowing oxygen carriers
- Suitable particle sizes and porosities
- Continuous process
- No side products generated.
3.6.4. Coprecipitation
3.6.5. Sol-Gel Synthesis
3.6.6. Impregnation
4. Industrial Applicability—Environmental Aspects and Cost
5. Future Outlook
- Further improving the long-term stability and maintaining the high activity of oxygen carriers.
- Developing novel oxygen carriers where optimal materials are combined and where the composition of the active phase is selected and modified in such a way that inherently a high selectivity can be obtained.
- Developing oxygen carriers synthesized with minimal cost, e.g., by starting from impure raw materials or by further developing routes from naturally occurring ores, and checking the effect of the actual use of relevant impure materials on the oxygen carrier performance.
- Developing novel oxygen carrier shapes that can be suitable for use inside pressurized reactor systems, where chemical looping has interesting opportunities, utilized integrated into a system.
- Optimizing oxygen carrier chemistry for use at higher pressures.
- Developing oxygen carriers suitable for catalyst-assisted chemical looping (e.g., see [61,292,293,295]). The authors expect that the interaction between catalysis and chemical looping will make significant changes to the chemical looping landscape in the following years and, because of its potential benefits, deserves extensive attention from the research community.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stocker, T.F.; Qin, G.-K.D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S.; et al. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- IPCC. 2014: Summary for Policymakers. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–30. [Google Scholar]
- Barros, V.R.; Mach, K.J.; Mastrandrea, M.D.; van Aalst, M.; Adger, W.N.; Arent, D.J.; Barnett, J.; Betts, R.; Bilir, T.E.; Birkmann, J.; et al. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Allen, M.R.; de Coninck, H.; Dube, O.P.; Hoegh-Guldberg, O.; Jacob, D.; Jiang, K.; Revi, A.; Rogelj, J.; Roy, J.; Shindell, D.; et al. 2018: Technical Summary. In Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Incheon, Korea, 2018; In Press. [Google Scholar]
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Kadner, S.; Minx, J.C.; Brunner, S.; Agrawala, S.; Baiocchi, G.; Bashmakov, I.A.; Blanco, G.; et al. 2014: Technical Summary. In Climate Change 2014: Mitigation of Climate Change; Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 33–107. [Google Scholar]
- European Commission. Analysis of Options beyond 20% GHG Emission Reductions: Member State Results; European Commission: Brussels, Belgium, 2012; pp. 1–49. [Google Scholar]
- Liu, Y.; Deng, S.; Zhao, R.; He, J.; Zhao, L. Energy-saving pathway exploration of CCS integrated with solar energy: A review of innovative concepts. Renew. Sustain. Energy Rev. 2017, 77, 652–669. [Google Scholar] [CrossRef]
- Leeson, D.; Mac Dowell, N.; Shah, N.; Petit, C.; Fennell, P.S. A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int. J. Greenh. Gas Control 2017, 61, 71–84. [Google Scholar] [CrossRef]
- Cormos, C.-C. Oxy-combustion of coal, lignite and biomass: A techno-economic analysis for a large scale Carbon Capture and Storage (CCS) project in Romania. Fuel 2016, 169, 50–57. [Google Scholar] [CrossRef]
- Cormos, C.-C. Evaluation of syngas-based chemical looping applications for hydrogen and power co-generation with CCS. Int. J. Hydrogen Energy 2012, 37, 13371–13386. [Google Scholar] [CrossRef]
- Cormos, C.-C. Evaluation of power generation schemes based on hydrogen-fuelled combined cycle with carbon capture and storage (CCS). Int. J. Hydrogen Energy 2011, 36, 3726–3738. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, R.H.S.; Fennell, P.; Livingston, W.R.; Shah, N.; Dowell, N.M.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M.; et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 2017, 190, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Körner, A.; Tam, C.; Bennett, S.; Gagné, J.F. Technology Roadmap Hydrogen and Fuel Cells; International Energy Agency (IEA): Paris, France, 2015. [Google Scholar]
- Muradov, N. Low to near-zero CO2 production of hydrogen from fossil fuels: Status and perspectives. Int. J. Hydrogen Energy 2017, 42, 14058–14088. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Zhu, L.; He, Y.; Li, L.; Wu, P. Tech-economic assessment of second-generation CCS: Chemical looping combustion. Energy 2018, 144, 915–927. [Google Scholar] [CrossRef]
- Whitty, K.; Wagner, D.R.; Backman, M.; Dobo, Z.; Merrett, K.M.; Dai, J. Experience with Chemical Looping Combustion of Coal in a 200 kWth Dual Fluidized Bed Reactor. In Proceedings of the 5th International conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018; p. 18. [Google Scholar]
- Gilliland, R.E.; Lewis, W.K. Production of Pure Carbon Dioxide. U.S. Patent 2,665,972, 13 November 1950. [Google Scholar]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayán, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Fang, H.; Haibin, L.; Zengli, Z. Advancements in Development of Chemical-Looping Combustion: A Review. Int. J. Chem. Eng. 2009, 2009, 1–16. [Google Scholar] [CrossRef]
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control 2015, 40, 126–166. [Google Scholar] [CrossRef] [Green Version]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of Chemical Looping Combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Johansson, M.; Mattisson, T. Chemical-Looping Combustion—Status of Development. In Proceedings of the 9th International Conference on Circulating Fluidized Beds (CFB-9), Hamburg, Germany, 13–16 May 2008; p. 16. [Google Scholar]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical-looping combustion of solid fuels—Status of development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar] [CrossRef] [Green Version]
- Lyngfelt, A.; Linderholm, C. Chemical-Looping Combustion of Solid Fuels—Status and Recent Progress. Energy Procedia 2017, 114, 371–386. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies 2015, 8, 10605–10635. [Google Scholar] [CrossRef] [Green Version]
- Lyngfelt, A. Oxygen Carriers for Chemical Looping Combustion—4000 h of Operational Experience. Oil Gas Sci. Technol. Rev. D’IFP Energ. Nouv. 2011, 66, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Moghtaderi, B. Review of the Recent Chemical Looping Process Developments for Novel Energy and Fuel Applications. Energy Fuels 2012, 26, 15–40. [Google Scholar] [CrossRef]
- Song, T.; Shen, L. Review of reactor for chemical looping combustion of solid fuels. Int. J. Greenh. Gas Control 2018, 76, 92–110. [Google Scholar] [CrossRef]
- Mishra, A.; Li, F. Chemical looping at the nanoscale—challenges and opportunities. Curr. Opin. Chem. Eng. 2018, 20, 143–150. [Google Scholar] [CrossRef]
- Mattisson, T.; Keller, M.; Linderholm, C.; Moldenhauer, P.; Rydén, M.; Leion, H.; Lyngfelt, A. Chemical-looping technologies using circulating fluidized bed systems: Status of development. Fuel Process. Technol. 2018, 172, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luo, M. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Fan, J.A.; Fan, L.-S. New Insight into the Development of Oxygen Carrier Materials for Chemical Looping Systems. Engineering 2018. [Google Scholar] [CrossRef]
- Matzen, M.; Pinkerton, J.; Wang, X.; Demirel, Y. Use of natural ores as oxygen carriers in chemical looping combustion: A review. Int. J. Greenh. Gas Control 2017, 65, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Batra, V.S.; Li, H.-P. Oxygen carrier materials and their role in chemical looping reactions for fuel conversion. Curr. Opin. Chem. Eng. 2017, 15, 44–48. [Google Scholar] [CrossRef]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Udomsirichakorn, J.; Salam, P.A. Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renew. Sustain. Energy Rev. 2014, 30, 565–579. [Google Scholar] [CrossRef]
- Pröll, T.; Kolbitsch, P.; Bolhàr-Nordenkampf, J.; Hofbauer, H. A novel dual circulating fluidized bed system for chemical looping processes. AIChE J. 2009, 55, 3255–3266. [Google Scholar] [CrossRef]
- Haus, J.; Lyu, K.; Hartge, E.-U.; Heinrich, S.; Werther, J. Analysis of a Two-Stage Fuel Reactor System for the Chemical-Looping Combustion of Lignite and Bituminous Coal. Energy Technol. 2016, 4, 1263–1273. [Google Scholar] [CrossRef]
- Markström, P.; Linderholm, C.; Lyngfelt, A. Chemical-looping combustion of solid fuels—Design and operation of a 100kW unit with bituminous coal. Int. J. Greenh. Gas Control 2013, 15, 150–162. [Google Scholar] [CrossRef]
- Ströhle, J.; Orth, M.; Epple, B. Design and operation of a 1MWth chemical looping plant. Appl. Energy 2014, 113, 1490–1495. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, L.; Saha, C.; Zhang, S.; Bhattacharya, S. Pressurized chemical-looping combustion of coal using an iron ore as oxygen carrier in a pilot-scale unit. Int. J. Greenh. Gas Control 2012, 10, 363–373. [Google Scholar] [CrossRef]
- Weber, J.; Straub, D.; Breault, R.W.; Richards, G. Operating Experience of a Chemical Looping Circulating Fluidized Bed Combustor. In Proceedings of the 39th International Technical Conference on Clean Coal & Fuel Systems, Clearwater, FL, USA, 1–5 June 2014. [Google Scholar]
- Abad, A.; Pérez-Vega, R.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Design and operation of a 50kWth Chemical Looping Combustion (CLC) unit for solid fuels. Appl. Energy 2015, 157, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhao, H.; Niu, P.; Chen, X.; Tian, X.; Zheng, C. Design and Operation of a 50 kWth Chemical Looping Combustion (CLC) Reactor using Coal as Fuel. In Proceedings of the 4th International Conference on Chemical Looping, Southeast University, Nanjing, China, 26–28 September 2016. [Google Scholar]
- Pikkarainen, T.; Hiltunen, I.; Tier, S. Piloting of Bio-CLC for BECCS. In Proceedings of the 4th International Conference on Chemical Looping, Nanjing, China, 26–28 September 2016. [Google Scholar]
- Lin, S.Y.; Saito, T. Development of Three-Tower (Reactors) Technology for Chemical Looping Coal Combustion. In Proceedings of the 4th International Conference on Chemical Looping, Nanjing, China, 26–28 September 2016. [Google Scholar]
- Ryu, H.; Lee, D.; Jang, M.; Kim, J.; Baek, J.-I. Conceptual Design and Feasibility Study on 0.5 MWth Pressurized Chemical Looping Combustor. Trans. Korean Hydrog. New Energy Soc. 2016, 27, 201–210. [Google Scholar] [CrossRef]
- Diglio, G.; Bareschino, P.; Mancusi, E.; Pepe, F. Novel quasi -autothermal hydrogen production process in a fixed-bed using a chemical looping approach: A numerical study. Int. J. Hydrogen Energy 2017, 42, 15010–15023. [Google Scholar] [CrossRef]
- Parishan, S.; Littlewood, P.; Arinchtein, A.; Fleischer, V.; Schomäcker, R. Chemical looping as a reactor concept for the oxidative coupling of methane over the MnxOy-Na2WO4/SiO2 catalyst, benefits and limitation. Catal. Today 2018, 311, 40–47. [Google Scholar] [CrossRef]
- Fleischer, V.; Littlewood, P.; Parishan, S.; Schomäcker, R. Chemical looping as reactor concept for the oxidative coupling of methane over a Na2WO4/Mn/SiO2 catalyst. Chem. Eng. J. 2016, 306, 646–654. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G.; Xia, C. Reaction schemes of barium ferrite in biomass chemical looping gasification for hydrogen-enriched syngas generation via an outer-inner looping redox reaction mechanism. Energy Convers. Manag. 2019, 189, 81–90. [Google Scholar] [CrossRef]
- Hosseini, S.Y.; Khosravi-Nikou, M.R.; Shariati, A. Production of hydrogen and syngas using chemical looping technology via cerium-iron mixed oxides. Chem. Eng. Process. Process Intensif. 2019, 139, 23–33. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Wang, Y.; Li, K.; Wang, Y.; Jiang, L.; Zhu, X.; Wei, Y.; Wang, H. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane. Appl. Surf. Sci. 2019, 481, 151–160. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Dunstan, M.T.; Scott, S.A. Development and performance of iron based oxygen carriers containing calcium ferrites for chemical looping combustion and production of hydrogen. Int. J. Hydrogen Energy 2016, 41, 4073–4084. [Google Scholar] [CrossRef] [Green Version]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B Environ. 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Wenzel, M.; Aditya Dharanipragada, N.V.R.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Rihko-Struckmann, L.; Sundmacher, K. CO production from CO2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. J. CO2 Util. 2017, 17, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.S.C.; Liu, W.; Ismail, M.; Yang, Y.; Scott, S.A.; Dennis, J.S. Improving hydrogen yields, and hydrogen:steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chem. Eng. J. 2016, 296, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Using chemical-looping with oxygen uncoupling (CLOU) for combustion of six different solid fuels. Energy Procedia 2009, 1, 447–453. [Google Scholar] [CrossRef] [Green Version]
- García-Labiano, F.; de Diego, L.F.; García-Díez, E.; Serrano, A.; Abad, A.; Gayán, P.; Adánez, J. Combustion and Reforming of Liquid Fossil Fuels through Chemical Looping Processes: Integration of Chemical Looping Processes in a Refinery. Energy Procedia 2017, 114, 325–333. [Google Scholar] [CrossRef]
- Rydén, M.; Moldenhauer, P.; Mattisson, T.; Lyngfelt, A.; Younes, M.; Niass, T.; Fadhel, B.; Ballaguet, J.-P. Chemical-Looping Combustion with Liquid Fuels. Energy Procedia 2013, 37, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Moldenhauer, P.; Rydén, M.; Mattisson, T.; Jamal, A.; Lyngfelt, A. Chemical-looping combustion with heavy liquid fuels in a 10kW pilot plant. Fuel Process. Technol. 2017, 156, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, V.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Exploring novel hydrogen production processes by integration of steam methane reforming with chemical-looping combustion (CLC-SMR) and oxygen carrier aided combustion (OCAC-SMR). Int. J. Greenh. Gas Control 2018, 74, 28–39. [Google Scholar] [CrossRef]
- Stollhof, M.; Penthor, S.; Mayer, K.; Hofbauer, H. Fluid dynamic evaluation of a 10 MW scale reactor design for chemical looping combustion of gaseous fuels. Chem. Eng. Sci. 2018, 178, 48–60. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, S.; Sridhar, D.; Fan, L.-S. Chemical looping processes—particle characterization, ionic diffusion-reaction mechanism and reactor engineering. Rev. Chem. Eng. 2012, 28, 1–42. [Google Scholar] [CrossRef]
- Noorman, S.; van Sint Annaland, M.; Kuipers, J.A.M. Experimental validation of packed bed chemical-looping combustion. Chem. Eng. Sci. 2010, 65, 92–97. [Google Scholar] [CrossRef]
- Ortiz, M.; Gallucci, F.; Snijkers, F.; Van Noyen, J.; Louradour, E.; Tournigant, D.; van Sint Annaland, M. Development and testing of ilmenite granules for packed bed chemical-looping combustion. Chem. Eng. J. 2014, 245, 228–240. [Google Scholar] [CrossRef]
- Spallina, V.; Gallucci, F.; Romano, M.C.; Chiesa, P.; Lozza, G.; van Sint Annaland, M. Investigation of heat management for CLC of syngas in packed bed reactors. Chem. Eng. J. 2013, 225, 174–191. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, L.; Cloete, S.; Chiesa, P.; Amini, S. Economic assessment of packed bed chemical looping combustion and suitable benchmarks. Int. J. Greenh. Gas Control 2017, 64, 223–233. [Google Scholar] [CrossRef]
- Zerobin, F.; Penthor, S.; Bertsch, O.; Pröll, T. Fluidized bed reactor design study for pressurized chemical looping combustion of natural gas. Powder Technol. 2017, 316, 569–577. [Google Scholar] [CrossRef]
- Dahl, I.M.; Bakken, E.; Larring, Y.; Spjelkavik, A.I.; Håkonsen, S.F.; Blom, R. On the development of novel reactor concepts for chemical looping combustion. Energy Procedia 2009, 1, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Hamers, H.P.; Gallucci, F.; Cobden, P.D.; Kimball, E.; van Sint Annaland, M. A novel reactor configuration for packed bed chemical-looping combustion of syngas. Int. J. Greenh. Gas Control 2013, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Zhu, J.; Wu, X.; Xia, Z.; Deng, Z.; Wang, W. Packed Bed Chemical Looping Platform: Design and Operation of 30kWth Pilot Unit. Procedia Environ. Sci. 2016, 31, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.; Van Noyen, J.; Larring, Y.; Mccann, M.; Pishahang, M.; Amini, S.; Ortiz, M.; Galluci, F.; Sint-Annaland, M.V.; Tournigant, D.; et al. Thermal and mechanical behaviour of oxygen carrier materials for chemical looping combustion in a packed bed reactor. Appl. Energy 2015, 157, 374–381. [Google Scholar] [CrossRef]
- Hamers, H.P.; Romano, M.C.; Spallina, V.; Chiesa, P.; Gallucci, F.; van Sint Annaland, M. Comparison on process efficiency for CLC of syngas operated in packed bed and fluidized bed reactors. Int. J. Greenh. Gas Control 2014, 28, 65–78. [Google Scholar] [CrossRef]
- Hamers, H.P.; Gallucci, F.; Cobden, P.D.; Kimball, E.; van Sint Annaland, M. CLC in packed beds using syngas and CuO/Al2O3: Model description and experimental validation. Appl. Energy 2014, 119, 163–172. [Google Scholar] [CrossRef]
- Spallina, V.; Marinello, B.; Gallucci, F.; Romano, M.C.; van Sint Annaland, M. Chemical looping reforming in packed-bed reactors: Modelling, experimental validation and large-scale reactor design. Fuel Process. Technol. 2017, 156, 156–170. [Google Scholar] [CrossRef]
- Zacharias, R.; Visentin, S.; Bock, S.; Hacker, V. High-pressure hydrogen production with inherent sequestration of a pure carbon dioxide stream via fixed bed chemical looping. Int. J. Hydrogen Energy 2019, 44, 7943–7957. [Google Scholar] [CrossRef]
- Chen, C.; Bollas, G.M. Design and Scheduling of Semibatch Chemical-Looping Reactors. Ind. Eng. Chem. Res. 2020, 59, 6994–7006. [Google Scholar] [CrossRef]
- Luzi, C.D.; Martínez, O.M.; Barreto, G.F. Autothermal reverse-flow reactors: Design and comparison of valve-operated and rotary systems. Chem. Eng. Sci. 2016, 148, 170–181. [Google Scholar] [CrossRef]
- Zhao, Z.; Iloeje, C.O.; Chen, T.; Ghoniem, A.F. Design of a rotary reactor for chemical-looping combustion. Part 1: Fundamentals and design methodology. Fuel 2014, 121, 327–343. [Google Scholar] [CrossRef]
- Zhao, Z.; Ghoniem, A.F. Design of a rotary reactor for chemical-looping combustion. Part 2: Comparison of copper-, nickel-, and iron-based oxygen carriers. Fuel 2014, 121, 344–360. [Google Scholar] [CrossRef]
- Iloeje, C.O.; Zhao, Z.; Ghoniem, A.F. A reduced fidelity model for the rotary chemical looping combustion reactor. Appl. Energy 2017, 190, 725–739. [Google Scholar] [CrossRef]
- Iloeje, C.O.; Zhao, Z.; Ghoniem, A.F. Design and techno-economic optimization of a rotary chemical looping combustion power plant with CO2 capture. Appl. Energy 2018, 231, 1179–1190. [Google Scholar] [CrossRef]
- Dennis, J.S.; Scott, S.A. In situ gasification of a lignite coal and CO2 separation using chemical looping with a Cu-based oxygen carrier. Fuel 2010, 89, 1623–1640. [Google Scholar] [CrossRef]
- Spallina, V.; Chiesa, P.; Martelli, E.; Gallucci, F.; Romano, M.C.; Lozza, G.; van Sint Annaland, M. Reactor design and operation strategies for a large-scale packed-bed CLC power plant with coal syngas. Int. J. Greenh. Gas Control 2015, 36, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Huang, Z. High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Velasco-Sarria, F.J.; Forero, C.R.; Adánez-Rubio, I.; Abad, A.; Adánez, J. Assessment of low-cost oxygen carrier in South-western Colombia, and its use in the in-situ gasification chemical looping combustion technology. Fuel 2018, 218, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Arjmand, M.; Leion, H.; Mattisson, T. Interaction of mineral matter of coal with oxygen carriers in chemical-looping combustion (CLC). Chem. Eng. Res. Des. 2013. [Google Scholar] [CrossRef]
- Azis, M.M.; Jerndal, E.; Leion, H.; Mattisson, T.; Lyngfelt, A. On the evaluation of synthetic and natural ilmenite using syngas as fuel in chemical-looping combustion (CLC). Chem. Eng. Res. Des. 2010, 88, 1505–1514. [Google Scholar] [CrossRef]
- Schmitz, M.; Linderholm, C. Chemical looping combustion of biomass in 10- and 100-kW pilots—Analysis of conversion and lifetime using a sintered manganese ore. Fuel 2018, 231, 73–84. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Hallberg, P.; Leion, H.; Lyngfelt, A. A method for determination of reaction enthalpy of oxygen carriers for chemical looping combustion—Application to ilmenite. Thermochim. Acta 2011, 524, 62–67. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Adánez, J.; Abad, A. Development of CuO-based oxygen-carrier materials suitable for Chemical-Looping with Oxygen Uncoupling (CLOU) process. Energy Procedia 2011, 4, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Shulman, A.; Cleverstam, E.; Mattisson, T.; Lyngfelt, A. Manganese/Iron, Manganese/Nickel, and Manganese/Silicon Oxides Used in Chemical-Looping With Oxygen Uncoupling (CLOU) for Combustion of Methane. Energy Fuels 2009, 23, 5269–5275. [Google Scholar] [CrossRef]
- Mattisson, T.; Linderholm, C.; Jerndal, E.; Lyngfelt, A. Enhanced performance of manganese ore as oxygen carrier for chemical-looping with oxygen uncoupling (CLOU) by combination with Ca(OH)2 through spray-drying. J. Environ. Chem. Eng. 2016, 4, 3707–3717. [Google Scholar] [CrossRef]
- Hallberg, P.; Hanning, M.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Investigation of a calcium manganite as oxygen carrier during 99 h of operation of chemical-looping combustion in a 10 kW th reactor unit. Int. J. Greenh. Gas Control 2016, 53, 222–229. [Google Scholar] [CrossRef]
- Zaabout, A.; Cloete, S.; van Sint Annaland, M.; Gallucci, F.; Amini, S. Experimental demonstration of control strategies for a Gas Switching Combustion reactor for power production with integrated CO2 capture. Chem. Eng. Res. Des. 2016, 111, 342–352. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Identification of operational regions in the Chemical-Looping with Oxygen Uncoupling (CLOU) process with a Cu-based oxygen carrier. Fuel 2012, 102, 634–645. [Google Scholar] [CrossRef] [Green Version]
- Ryden, M.; Lyngfelt, A. Using steam reforming to produce hydrogen with carbon dioxide capture by chemical-looping combustion. Int. J. Hydrogen Energy 2006, 31, 1271–1283. [Google Scholar] [CrossRef] [Green Version]
- García-Díez, E.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Gayán, P.; Adánez, J.; Ruíz, J.A.C. Optimization of hydrogen production with CO2 capture by autothermal chemical-looping reforming using different bioethanol purities. Appl. Energy 2016, 169, 491–498. [Google Scholar] [CrossRef]
- Ortiz, M.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Optimization of hydrogen production by Chemical-Looping auto-thermal Reforming working with Ni-based oxygen-carriers. Int. J. Hydrogen Energy 2011, 36, 9663–9672. [Google Scholar] [CrossRef] [Green Version]
- Francisco Morgado, J.; Cloete, S.; Morud, J.; Gurker, T.; Amini, S. Modelling study of two chemical looping reforming reactor configurations: Looping vs. switching. Powder Technol. 2017, 316, 599–613. [Google Scholar] [CrossRef]
- Wassie, S.A.; Gallucci, F.; Zaabout, A.; Cloete, S.; Amini, S.; van Sint Annaland, M. Hydrogen production with integrated CO2 capture in a novel gas switching reforming reactor: Proof-of-concept. Int. J. Hydrogen Energy 2017, 42, 14367–14379. [Google Scholar] [CrossRef]
- Zaabout, A.; Cloete, S.; Tolchard, J.R.; Amini, S. A pressurized Gas Switching Combustion reactor: Autothermal operation with a CaMnO3-based oxygen carrier. Chem. Eng. Res. Des. 2018, 137, 20–32. [Google Scholar] [CrossRef]
- Khan, M.N.; Shamim, T. Thermodynamic screening of suitable oxygen carriers for a three reactor chemical looping reforming system. Int. J. Hydrogen Energy 2017, 42, 15745–15760. [Google Scholar] [CrossRef]
- Khan, M.N.; Shamim, T. Investigation of hydrogen generation in a three reactor chemical looping reforming process. Appl. Energy 2016, 162, 1186–1194. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, W.; Xue, Z.; Sun, X. Experimental investigation of chemical looping hydrogen generation using iron oxides in a batch fluidized bed. Proc. Combust. Inst. 2011, 33, 2691–2699. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H. Thermodynamic analysis of chemical-looping hydrogen generation. Appl. Energy 2013, 112, 800–807. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, N.; Guo, X. Density functional theory study on improved reactivity of alkali-doped Fe2O3 oxygen carriers for chemical looping hydrogen production. Fuel 2019, 236, 1057–1064. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Soomro, A.; Hu, J.; Sun, Z.; Ma, S.; Xiang, W. Effects of supports on reduction activity and carbon deposition of iron oxide for methane chemical looping hydrogen generation. Appl. Energy 2018, 225, 912–921. [Google Scholar] [CrossRef]
- Ma, S.; Li, M.; Wang, G.; Zhang, L.; Chen, S.; Sun, Z.; Hu, J.; Zhu, M.; Xiang, W. Effects of Zr doping on Fe2O3/CeO2 oxygen carrier in chemical looping hydrogen generation. Chem. Eng. J. 2018, 346, 712–725. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Soomro, A.; Zhu, M.; Xiang, W. Characterization of Fe2O3/CeO2 oxygen carriers for chemical looping hydrogen generation. Int. J. Hydrogen Energy 2018, 43, 3154–3164. [Google Scholar] [CrossRef]
- Kathe, M.V.; Empfield, A.; Na, J.; Blair, E.; Fan, L.-S. Hydrogen production from natural gas using an iron-based chemical looping technology: Thermodynamic simulations and process system analysis. Appl. Energy 2016, 165, 183–201. [Google Scholar] [CrossRef]
- Messerschmitt, A. Process of Producing Hydrogen. U.S. Patent 971,206, 2 December 1908. [Google Scholar]
- Wassie, S.A.; Gallucci, F.; Cloete, S.; Zaabout, A.; van Sint Annaland, M.; Amini, S. The effect of gas permeation through vertical membranes on chemical switching reforming (CSR) reactor performance. Int. J. Hydrogen Energy 2016, 41, 8640–8655. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Smith, A.R.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Hashim, S.S.; Mohamed, A.R.; Bhatia, S. Oxygen separation from air using ceramic-based membrane technology for sustainable fuel production and power generation. Renew. Sustain. Energy Rev. 2011, 15, 1284–1293. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, B.; Zhao, Y.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z. Process simulation and thermodynamic evaluation for chemical looping air separation using fluidized bed reactors. Energy Convers. Manag. 2018, 160, 289–301. [Google Scholar] [CrossRef]
- Wu, F.; Argyle, M.D.; Dellenback, P.A.; Fan, M. Progress in O2 separation for oxy-fuel combustion–A promising way for cost-effective CO2 capture: A review. Prog. Energy Combust. Sci. 2018, 67, 188–205. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Qin, Q.; Zuo, Z.; Wu, T. Evaluation of Cu-based oxygen carrier for chemical looping air separation in a fixed-bed reactor. Chem. Eng. J. 2016, 287, 292–301. [Google Scholar] [CrossRef]
- Tian, X.; Wei, Y.; Zhao, H. Using a hierarchically-structured CuO@TiO2-Al2O3 oxygen carrier for chemical looping air separation in a paralleled fluidized bed reactor. Chem. Eng. J. 2018, 334, 611–618. [Google Scholar] [CrossRef]
- Ku, Y.; Wu, H.-C.; Chang, C.-W.; Shiu, S.-H. Chemical looping with air separation (CLAS) in a moving bed reactor with CuO/ZrO2 oxygen carriers. Int. J. Greenh. Gas Control 2018, 70, 225–235. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q. Long-lasting investigation of the Cu-based oxygen carrier particles in chemical looping air separation. Powder Technol. 2019, 343, 40–48. [Google Scholar] [CrossRef]
- Kimball, E.; Lambert, A.; Fossdal, A.; Leenman, R.; Comte, E.; van den Bos, W.A.P.; Blom, R. Reactor choices for chemical looping combustion (CLC)—Dependencies on materials characteristics. Energy Procedia 2013, 37, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Johansson, M. The use of iron oxide as oxygen carrier in a chemical-looping reactor. Fuel 2007, 86, 1021–1035. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Ryden, M. Chemical-looping combustion in a 300W continuously operating reactor system using a manganese-based oxygen carrier. Fuel 2006, 85, 1174–1185. [Google Scholar] [CrossRef]
- Costa, T.R.; Gayán, P.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Melo, D.M.A.; Adánez, J. Mn-based oxygen carriers prepared by impregnation for Chemical Looping Combustion with diverse fuels. Fuel Process. Technol. 2018, 178, 236–250. [Google Scholar] [CrossRef]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal Analysis of Chemical-Looping Combustion. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Azimi, G.; Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. (MnzFe1−z)yOx combined oxides as oxygen carrier for chemical-looping with oxygen uncoupling. AIChE J. 2013, 59, 582–588. [Google Scholar] [CrossRef]
- Cao, Y. Preparation and Characterization of Lanthanum-Promoted Copper-based Oxygen Carriers for Chemical Looping Combustion Process. Aerosol Air Qual. Res. 2014. [Google Scholar] [CrossRef] [Green Version]
- Haider, S.K.; Azimi, G.; Duan, L.; Anthony, E.J.; Patchigolla, K.; Oakey, J.E.; Leion, H.; Mattisson, T.; Lyngfelt, A. Enhancing properties of iron and manganese ores as oxygen carriers for chemical looping processes by dry impregnation. Appl. Energy 2016, 163, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Forero, C.R.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Adánez, J. High temperature behaviour of a CuO/γAl2O3 oxygen carrier for chemical-looping combustion. Int. J. Greenh. Gas Control 2011, 5, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; He, T.; Wang, Z.; Zhu, M.; Zhang, K.; Li, B.; Wu, J. The search of proper oxygen carriers for chemical looping partial oxidation of carbon. Appl. Energy 2017, 190, 1119–1125. [Google Scholar] [CrossRef]
- Yang, J.; Wei, Y.; Yang, J.; Xiang, H.; Ma, L.; Zhang, W.; Wang, L.; Peng, Y.; Liu, H. Syngas production by chemical looping gasification using Fe supported on phosphogypsum compound oxygen carrier. Energy 2019, 168, 126–135. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Källén, M.; Hallberg, P.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Combined oxides of iron, manganese and silica as oxygen carriers for chemical-looping combustion. Fuel Process. Technol. 2014, 124, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Moldenhauer, P.; Rydén, M.; Lyngfelt, A. Testing of minerals and industrial by-products as oxygen carriers for chemical-looping combustion in a circulating fluidized-bed 300W laboratory reactor. Fuel 2012, 93, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Kwak, B.S.; Park, N.-K.; Ryu, S.O.; Baek, J.-I.; Ryu, H.-J.; Kang, M. Improved reversible redox cycles on MTiOx (M = Fe, Co, Ni, and Cu) particles afforded by rapid and stable oxygen carrier capacity for use in chemical looping combustion of methane. Chem. Eng. J. 2017, 309, 617–627. [Google Scholar] [CrossRef]
- Adánez, J.; Garcá-Labiano, F.; Gayán, P.; de Diego, L.F.; Abad, A.; Dueso, C.; Forero, C.R. Effect of gas impurities on the behavior of Ni-based oxygen carriers on chemical-looping combustion. Energy Procedia 2009, 1, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Hong, H.; Zhu, L.; Wang, Z.; Jin, H. Thermodynamic evaluation of chemical looping combustion for combined cooling heating and power production driven by coal. Energy Convers. Manag. 2017, 135, 200–211. [Google Scholar] [CrossRef]
- Sharma, R.; May, J.; Alobaid, F.; Ohlemüller, P.; Ströhle, J.; Epple, B. Euler-Euler CFD simulation of the fuel reactor of a 1 MWth chemical-looping pilot plant: Influence of the drag models and specularity coefficient. Fuel 2017, 200, 435–446. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Mattisson, T. Solid fuels in chemical-looping combustion using a NiO-based oxygen carrier. Chem. Eng. Res. Des. 2009, 87, 1543–1550. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A. Chemical-looping combustion: Status and research needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar] [CrossRef]
- Arjmand, M.; Kooiman, R.F.; Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Sulfur Tolerance of CaxMn1–yMyO3−δ (M = Mg, Ti) Perovskite-Type Oxygen Carriers in Chemical-Looping with Oxygen Uncoupling (CLOU). Energy Fuels 2014, 28, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- Linderholm, C.; Knutsson, P.; Schmitz, M.; Markström, P.; Lyngfelt, A. Material balances of carbon, sulfur, nitrogen and ilmenite in a 100kW CLC reactor system. Int. J. Greenh. Gas Control 2014, 27, 188–202. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Rydén, M.; Mattisson, T.; Younes, M.; Lyngfelt, A. The use of ilmenite as oxygen carrier with kerosene in a 300W CLC laboratory reactor with continuous circulation. Appl. Energy 2014, 113, 1846–1854. [Google Scholar] [CrossRef] [Green Version]
- García-Labiano, F.; de Diego, L.F.; Gayán, P.; Abad, A.; Cabello, A.; Adánez, J.; Sprachmann, G. Energy exploitation of acid gas with high H2S content by means of a chemical looping combustion system. Appl. Energy 2014, 136, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.; Briault, P.; Comte, E. Spinel mixed oxides as oxygen carriers for chemical looping combustion. Energy Procedia 2011, 4, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Chen, Y.; Galinsky, N.; Wang, Z.; Li, F. Investigation of perovskite supported composite oxides for chemical looping conversion of syngas. Fuel 2014, 134, 521–530. [Google Scholar] [CrossRef]
- Galinsky, N.L.; Shafiefarhood, A.; Chen, Y.; Neal, L.; Li, F. Effect of support on redox stability of iron oxide for chemical looping conversion of methane. Appl. Catal. B Environ. 2015, 164, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Shafiefarhood, A.; Stewart, A.; Li, F. Iron-containing mixed-oxide composites as oxygen carriers for Chemical Looping with Oxygen Uncoupling (CLOU). Fuel 2015, 139, 1–10. [Google Scholar] [CrossRef]
- Bhavsar, S.; Isenberg, N.; More, A.; Veser, G. Lanthana-doped ceria as active support for oxygen carriers in chemical looping combustion. Appl. Energy 2016, 168, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Lee, M.; Lim, H.S.; Lee, J.W. Chemical looping partial oxidation of methane with CO2 utilization on the ceria-enhanced mesoporous Fe2O3 oxygen carrier. Fuel 2018, 215, 787–798. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Syngas production from methane and air via a redox process using Ce–Fe mixed oxides as oxygen carriers. Appl. Catal. B Environ. 2010, 97, 361–372. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Tang, L.; Wong, L.; Burke, N.; Trimm, D.; Nguyen, K.; Chiang, K. Hydrogen production through methane–steam cyclic redox processes with iron-based metal oxides. Int. J. Hydrogen Energy 2011, 36, 6646–6656. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, L.; Zheng, Y.; Wang, H.; Wei, Y.; Li, K. CeO2 modified Fe2O3 for the chemical hydrogen storage and production via cyclic water splitting. Int. J. Hydrogen Energy 2014, 39, 13381–13388. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Redox Investigation of Some Oxides of Transition-State Metals Ni, Cu, Fe, and Mn Supported on SiO2 and MgAl2O4. Energy Fuels 2006, 20, 34–44. [Google Scholar] [CrossRef]
- Wang, B.; Gao, C.; Wang, W.; Zhao, H.; Zheng, C. Sulfur evolution in chemical looping combustion of coal with MnFe2O4 oxygen carrier. J. Environ. Sci. 2014, 26, 1062–1070. [Google Scholar] [CrossRef]
- Ilyushechkin, A.Y.; Kochanek, M.; Lim, S. Interactions between oxygen carriers used for chemical looping combustion and ash from brown coals. Fuel Process. Technol. 2016, 147, 71–82. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Integrated Hydrogen and Power Production with CO2 Capture Using Chemical-Looping ReformingRedox Reactivity of Particles of CuO, Mn2O3, NiO, and Fe2O3 Using SiO2 as a Support. Ind. Eng. Chem. Res. 2005, 44, 3485–3496. [Google Scholar] [CrossRef]
- Zafar, Q.; Abad, A.; Mattisson, T.; Gevert, B.; Strand, M. Reduction and oxidation kinetics of Mn3O4/Mg–ZrO2 oxygen carrier particles for chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 6556–6567. [Google Scholar] [CrossRef]
- Jing, D.; Arjmand, M.; Mattisson, T.; Rydén, M.; Snijkers, F.; Leion, H.; Lyngfelt, A. Examination of oxygen uncoupling behaviour and reactivity towards methane for manganese silicate oxygen carriers in chemical-looping combustion. Int. J. Greenh. Gas Control 2014, 29, 70–81. [Google Scholar] [CrossRef]
- Jing, D.; Jacobs, M.; Hallberg, P.; Lyngfelt, A.; Mattisson, T. Development of CaMn0.775Mg0.1Ti0.125O3−δ oxygen carriers produced from different Mn and Ti sources. Mater. Des. 2016, 89, 527–542. [Google Scholar] [CrossRef]
- Källén, M.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Operation with Combined Oxides of Manganese and Silica as Oxygen Carriers in a 300 Wth Chemical-looping Combustion Unit. Energy Procedia 2014, 63, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Cuadrat, A.; Abad, A.; Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P. Behavior of ilmenite as oxygen carrier in chemical-looping combustion. Fuel Process. Technol. 2012, 94, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Wang, N.; Guo, X. Influence mechanism of supports on the reactivity of Ni-based oxygen carriers for chemical looping reforming: A DFT study. Fuel 2018, 229, 88–94. [Google Scholar] [CrossRef]
- Son, S.R.; Kim, S.D. Chemical-Looping Combustion with NiO and Fe2O3 in a Thermobalance and Circulating Fluidized Bed Reactor with Double Loops. Ind. Eng. Chem. Res. 2006, 45, 2689–2696. [Google Scholar] [CrossRef]
- Adánez, J.; Dueso, C.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A. Methane Combustion in a 500 Wth Chemical-Looping Combustion System Using an Impregnated Ni-Based Oxygen Carrier. Energy Fuels 2009, 23, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Corbella, B.M.; de Diego, L.F.; García-Labiano, F.; Adánez, J.; Palacios, J.M. Characterization Study and Five-Cycle Tests in a Fixed-Bed Reactor of Titania-Supported Nickel Oxide as Oxygen Carriers for the Chemical-Looping Combustion of Methane. Environ. Sci. Technol. 2005, 39, 5796–5803. [Google Scholar] [CrossRef]
- Abián, M.; Abad, A.; Izquierdo, M.T.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Titanium substituted manganese-ferrite as an oxygen carrier with permanent magnetic properties for chemical looping combustion of solid fuels. Fuel 2017, 195, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Combined oxides as oxygen-carrier material for chemical-looping with oxygen uncoupling. Appl. Energy 2014, 113, 1924–1932. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Neal, L.; Haribal, V.; Baldwin, M.; Lamb, H.H.; Li, F. Manganese silicate based redox catalysts for greener ethylene production via chemical looping—oxidative dehydrogenation of ethane. Appl. Catal. B Environ. 2018, 232, 77–85. [Google Scholar] [CrossRef]
- Costa, T.R.; Gayán, P.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Melo, D.M.A.; Adánez, J. Promising Impregnated Mn-based Oxygen Carriers for Chemical Looping Combustion of Gaseous Fuels. Energy Procedia 2017, 114, 334–343. [Google Scholar] [CrossRef]
- Gayán, P.; Adánez-Rubio, I.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Development of Cu-based oxygen carriers for Chemical-Looping with Oxygen Uncoupling (CLOU) process. Fuel 2012, 96, 226–238. [Google Scholar] [CrossRef]
- Kosaka, F.; Hatano, H.; Oshima, Y.; Otomo, J. Iron oxide redox reaction with oxide ion conducting supports for hydrogen production and storage systems. Chem. Eng. Sci. 2015, 123, 380–387. [Google Scholar] [CrossRef]
- Mattisson, T.; Leion, H.; Lyngfelt, A. Chemical-looping with oxygen uncoupling using CuO/ZrO2 with petroleum coke. Fuel 2009, 88, 683–690. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, L.; Wang, L.; Fu, K.; Tang, M.; Fan, M.; Ma, X. Syngas Production from Chemical-Looping Reforming of Methane Using Iron-Doped Cerium Oxides. Energy Technol. 2018, 6, 1610–1617. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Li, K.; Wei, Y.; Zheng, Y.; Hu, J.; Wang, H. Chemical-looping water splitting over ceria-modified iron oxide: Performance evolution and element migration during redox cycling. Chem. Eng. Sci. 2018, 179, 92–103. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Transformation of methane into synthesis gas using the redox property of Ce-Fe mixed oxides: Effect of calcination temperature. Int. J. Hydrogen Energy 2011, 36, 3471–3482. [Google Scholar] [CrossRef]
- Huang, H.B.; Aisyah, L.; Ashman, P.J.; Leung, Y.C.; Kwong, C.W. Chemical looping combustion of biomass-derived syngas using ceria-supported oxygen carriers. Bioresour. Technol. 2013, 140, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Siriwardane, R. Mechanism of Methane Chemical Looping Combustion with Hematite Promoted with CeO2. Energy Fuels 2013, 27, 4087–4096. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Driessche, I.; Van Der Voort, P.; Snijkers, F.; Verberckmoes, A. Processing and characterization of Fe-based oxygen carriers for chemical looping for hydrogen production. Int. J. Greenh. Gas Control 2018, 70, 12–21. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Sustainable iron-based oxygen carriers for Chemical Looping for Hydrogen Generation. Int. J. Hydrogen Energy 2019, 44, 1374–1391. [Google Scholar] [CrossRef]
- Cabello, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled Chemical Looping Combustion. Chem. Eng. J. 2014, 258, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Chemical looping combustion of ultra low concentration of methane with Fe2O3/Al2O3 and CuO/SiO2. Appl. Energy 2014, 113, 1916–1923. [Google Scholar] [CrossRef]
- Najera, M.; Solunke, R.; Gardner, T.; Veser, G. Carbon capture and utilization via chemical looping dry reforming. Chem. Eng. Res. Des. 2011, 89, 1533–1543. [Google Scholar] [CrossRef]
- Svoboda, K.; Slowinski, G.; Rogut, J.; Baxter, D. Thermodynamic possibilities and constraints for pure hydrogen production by iron based chemical looping process at lower temperatures. Energy Convers. Manag. 2007, 48, 3063–3073. [Google Scholar] [CrossRef]
- Cabello, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Izquierdo, M.T.; Adánez, J. Relevance of the catalytic activity on the performance of a NiO/CaAl2O4 oxygen carrier in a CLC process. Appl. Catal. B Environ. 2014, 147, 980–987. [Google Scholar] [CrossRef]
- Johansson, E.; Mattisson, T.; Lyngfelt, A.; Thunman, H. Combustion of Syngas and Natural Gas in a 300 W Chemical-Looping Combustor. Chem. Eng. Res. Des. 2006, 84, 819–827. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. Fundamental Study on a Novel Gas Turbine Cycle. J. Energy Resour. Technol. 2001, 123, 10. [Google Scholar] [CrossRef]
- Gayán, P.; Cabello, A.; García-Labiano, F.; Abad, A.; de Diego, L.F.; Adánez, J. Performance of a low Ni content oxygen carrier for fuel gas combustion in a continuous CLC unit using a CaO/Al2O3 system as support. Int. J. Greenh. Gas Control 2013, 14, 209–219. [Google Scholar] [CrossRef]
- Haider, S.K.; Erans, M.; Donat, F.; Duan, L.; Scott, S.A.; Manovic, V.; Anthony, E.J. Copper-based oxygen carriers supported with alumina/lime for the chemical looping conversion of gaseous fuels. J. Energy Chem. 2017, 26, 891–901. [Google Scholar] [CrossRef] [Green Version]
- Van Garderen, N.; Otal, E.H.; Aneziris, C.G.; Graule, T.; Clemens, F.J. Influence of porous substrate on copper based oxygen carrier efficiency for chemical-looping combustion. Microporous Mesoporous Mater. 2014, 190, 362–370. [Google Scholar] [CrossRef]
- San Pio, M.A.; Martini, M.; Gallucci, F.; Roghair, I.; van Sint Annaland, M. Kinetics of CuO/SiO2 and CuO/Al2O3 oxygen carriers for chemical looping combustion. Chem. Eng. Sci. 2018, 175, 56–71. [Google Scholar] [CrossRef]
- Arjmand, M.; Azad, A.-M.; Leion, H.; Lyngfelt, A.; Mattisson, T. Prospects of Al2O3 and MgAl2O4-Supported CuO Oxygen Carriers in Chemical-Looping Combustion (CLC) and Chemical-Looping with Oxygen Uncoupling (CLOU). Energy Fuels 2011, 25, 5493–5502. [Google Scholar] [CrossRef] [Green Version]
- Imtiaz, Q.; Broda, M.; Müller, C.R. Structure–property relationship of co-precipitated Cu-rich, Al2O3- or MgAl2O4-stabilized oxygen carriers for chemical looping with oxygen uncoupling (CLOU). Appl. Energy 2014, 119, 557–565. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Kurlov, A.; Rupp, J.S.; Müller, C.R. Highly Efficient Oxygen-Storage Material with Intrinsic Coke Resistance for Chemical Looping Combustion-Based CO2 Capture. ChemSusChem 2015, 8, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Mattisson, T.; Järdnäs, A.; Lyngfelt, A. Reactivity of Some Metal Oxides Supported on Alumina with Alternating Methane and OxygenApplication for Chemical-Looping Combustion. Energy Fuels 2003, 17, 643–651. [Google Scholar] [CrossRef]
- Shah, K.; Moghtaderi, B.; Wall, T. Selection of Suitable Oxygen Carriers for Chemical Looping Air Separation: A Thermodynamic Approach. Energy Fuels 2012, 26, 2038–2045. [Google Scholar] [CrossRef]
- Villa, R. Ni based mixed oxide materials for CH4 oxidation under redox cycle conditions. J. Mol. Catal. Chem. 2003, 204–205, 637–646. [Google Scholar] [CrossRef]
- Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J.; Abad, A.; Dueso, C. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemical-looping combustion. Fuel 2008, 87, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Erri, P.; Varma, A. Solution combustion synthesized oxygen carriers for chemical looping combustion. Chem. Eng. Sci. 2007, 62, 5682–5687. [Google Scholar] [CrossRef]
- Linderholm, C.; Mattisson, T.; Lyngfelt, A. Long-term integrity testing of spray-dried particles in a 10-kW chemical-looping combustor using natural gas as fuel. Fuel 2009, 88, 2083–2096. [Google Scholar] [CrossRef]
- Kuusik, R.; Trikkel, A.; Lyngfelt, A.; Mattisson, T. High temperature behavior of NiO-based oxygen carriers for Chemical Looping Combustion. Energy Procedia 2009, 1, 3885–3892. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Fernández, J.R.; Abanades, J.C.; Murillo, R.; Grasa, G. Conceptual design of a hydrogen production process from natural gas with CO2 capture using a Ca-Cu chemical loop. Int. J. Greenh. Gas Control 2012, 6, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Jerndal, E.; Mattisson, T.; Thijs, I.; Snijkers, F.; Lyngfelt, A. NiO particles with Ca and Mg based additives produced by spray- drying as oxygen carriers for chemical-looping combustion. Energy Procedia 2009, 1, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Mattisson, T.; Lyngfelt, A.; Abad, A. Using continuous and pulse experiments to compare two promising nickel-based oxygen carriers for use in chemical-looping technologies. Fuel 2008, 87, 988–1001. [Google Scholar] [CrossRef]
- Baek, J.-I.; Ryu, J.; Lee, J.B.; Eom, T.-H.; Kim, K.-S.; Yang, S.-R.; Ryu, C.K. Highly attrition resistant oxygen carrier for chemical looping combustion. Energy Procedia 2011, 4, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.-I.; Lee, J.B.; Eom, T.-H.; Kim, K.-S.; Yang, S.-R.; Ryu, C.K. Characterization of spray-dried NiO oxygen carriers supported on alpha alumina. Energy Procedia 2013, 37, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S.H. Hydrogen production via chemical looping steam methane reforming process: Effect of cerium and calcium promoters on the performance of Fe2O3/Al2O3 oxygen carrier. Appl. Energy 2016, 165, 685–694. [Google Scholar] [CrossRef]
- Luo, M.; Wang, S.; Wang, L.; Lv, M. Reduction kinetics of iron-based oxygen carriers using methane for chemical-looping combustion. J. Power Sources 2014, 270, 434–440. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. The use of petroleum coke as fuel in chemical-looping combustion. Fuel 2007, 86, 1947–1958. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar] [CrossRef]
- Rydén, M.; Arjmand, M. Continuous hydrogen production via the steam–iron reaction by chemical looping in a circulating fluidized-bed reactor. Int. J. Hydrogen Energy 2012, 37, 4843–4854. [Google Scholar] [CrossRef]
- De Vos, Y.; Vamvakeros, A.; Matras, D.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Jacques, S.; Middelkoop, V.; Verberckmoes, A. Sustainable iron-based oxygen carriers for hydrogen production—Real-time operando investigation. Int. J. Greenh. Gas Control 2019, 88, 393–402. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.; Hu, J.; Chen, A.; Rony, A.H.; Russell, C.K.; Xiang, W.; Fan, M.; Darby Dyar, M.; Dklute, E.C. Ca2Fe2O5: A promising oxygen carrier for CO/CH4 conversion and almost-pure H2 production with inherent CO2 capture over a two-step chemical looping hydrogen generation process. Appl. Energy 2018, 211, 431–442. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.; Russell, C.K.; Hu, J.; Rony, A.H.; Tan, G.; Chen, A.; Duan, L.; Boman, J.; Tang, J.; et al. Improvement of H2-rich gas production with tar abatement from pine wood conversion over bi-functional Ca2Fe2O5 catalyst: Investigation of inner-looping redox reaction and promoting mechanisms. Appl. Energy 2018, 212, 931–943. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Hsu, W.-M.; Chiu, P.-C.; Tseng, Y.-H.; Ku, Y. Assessment of redox behavior of nickel ferrite as oxygen carriers for chemical looping process. Ceram. Int. 2013, 39, 5459–5465. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X. Application of calcium ferrites as oxygen carriers for microalgae chemical looping gasification. Energy Convers. Manag. 2018, 160, 262–272. [Google Scholar] [CrossRef]
- Lachén, J.; Durán, P.; Peña, J.A.; Herguido, J. High purity hydrogen from coupled dry reforming and steam iron process with cobalt ferrites as oxygen carrier: Process improvement with the addition of NiAl2O4 catalyst. Catal. Today 2017. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-type oxides LaFe1−xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production. Appl. Energy 2016, 168, 193–203. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, J.; Li, Z.; Liu, M.; Ma, Y.; Zhang, X. Reduction kinetics of lanthanum ferrite perovskite for the production of synthesis gas by chemical-looping methane reforming. Chem. Eng. Sci. 2016, 153, 236–245. [Google Scholar] [CrossRef]
- Zhao, K.; Shen, Y.; Huang, Z.; He, F.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Different oxidation routes for lattice oxygen recovery of double-perovskite type oxides LaSrFeCoO6 as oxygen carriers for chemical looping steam methane reforming. J. Energy Chem. 2017, 26, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Song, Y.; Jiang, B.; Wang, K.; Zhang, Q. A novel oxygen carrier for chemical looping reforming: LaNiO3 perovskite supported on montmorillonite. Energy 2017, 131, 58–66. [Google Scholar] [CrossRef]
- Galinsky, N.; Sendi, M.; Bowers, L.; Li, F. CaMn1−xBxO3−δ(B = Al, V, Fe, Co, and Ni) perovskite based oxygen carriers for chemical looping with oxygen uncoupling (CLOU). Appl. Energy 2016, 174, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Dueso, C.; Thompson, C.; Metcalfe, I. High-stability, high-capacity oxygen carriers: Iron oxide-perovskite composite materials for hydrogen production by chemical looping. Appl. Energy 2015, 157, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Mattisson, T.; Jing, D.; Lyngfelt, A.; Rydén, M. Experimental investigation of binary and ternary combined manganese oxides for chemical-looping with oxygen uncoupling (CLOU). Fuel 2016, 164, 228–236. [Google Scholar] [CrossRef]
- Mattisson, T. Materials for Chemical-Looping with Oxygen Uncoupling. ISRN Chem. Eng. 2013, 2013, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, P.; Jing, D.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Chemical Looping Combustion and Chemical Looping with Oxygen Uncoupling Experiments in a Batch Reactor Using Spray-Dried CaMn1–xMxO3−δ(M = Ti, Fe, Mg) Particles as Oxygen Carriers. Energy Fuels 2013, 27, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, P.; Källén, M.; Jing, D.; Snijkers, F.; van Noyen, J.; Rydén, M.; Lyngfelt, A. Experimental Investigation of CaMnO3−δ Based Oxygen Carriers Used in Continuous Chemical-Looping Combustion. Int. J. Chem. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Liang, H. Study on the effect of CeO2 on Fe2O3/LaNiO3 as the oxygen carrier applied in chemical-looping hydrogen generation. Int. J. Hydrogen Energy 2015, 40, 13338–13343. [Google Scholar] [CrossRef]
- Arjmand, M.; Azad, A.-M.; Leion, H.; Rydén, M.; Mattisson, T. CaZrO3 and SrZrO3-based CuO Oxygen Carriers for Chemical-looping with Oxygen Uncoupling (CLOU). Energy Procedia 2014, 51, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X. Reactivity of Co-doped Ca2Fe2O5 brownmillerite oxides as oxygen carriers for microalgae chemical looping gasification. Int. J. Hydrogen Energy 2019, 44, 2546–2559. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X. Evaluation of Sr-substituted Ca2Fe2O5 as oxygen carrier in microalgae chemical looping gasification. Fuel Process. Technol. 2019, 191, 93–103. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Miller, D.D.; Siriwardane, R. CaFe2O4 oxygen carrier characterization during the partial oxidation of coal in the chemical looping gasification application. Appl. Energy 2018, 224, 708–716. [Google Scholar] [CrossRef]
- Riley, J.; Siriwardane, R.; Tian, H.; Benincosa, W.; Poston, J. Kinetic analysis of the interactions between calcium ferrite and coal char for chemical looping gasification applications: Identifying reduction routes and modes of oxygen transfer. Appl. Energy 2017, 201, 94–110. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Carbon Formation on Nickel and Iron Oxide-Containing Oxygen Carriers for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2005, 44, 668–676. [Google Scholar] [CrossRef]
- Galinsky, N.L.; Huang, Y.; Shafiefarhood, A.; Li, F. Iron Oxide with Facilitated O2– Transport for Facile Fuel Oxidation and CO2 Capture in a Chemical Looping Scheme. ACS Sustain. Chem. Eng. 2013, 1, 364–373. [Google Scholar] [CrossRef]
- Cabello, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Adánez, J. On the attrition evaluation of oxygen carriers in Chemical Looping Combustion. Fuel Process. Technol. 2016, 148, 188–197. [Google Scholar] [CrossRef]
- Rydén, M.; Moldenhauer, P.; Lindqvist, S.; Mattisson, T.; Lyngfelt, A. Measuring attrition resistance of oxygen carrier particles for chemical looping combustion with a customized jet cup. Powder Technol. 2014, 256, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Investigation of Fe2O3 with MgAl2O4 for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2004, 43, 6978–6987. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Izquierdo, M.T.; Adánez, J. Chemical Looping Combustion of gaseous and solid fuels with manganese-iron mixed oxide as oxygen carrier. Energy Convers. Manag. 2018, 159, 221–231. [Google Scholar] [CrossRef]
- Shulman, A.; Cleverstam, E.; Mattisson, T.; Lyngfelt, A. Chemical-Looping with oxygen uncoupling using Mn/Mg-based oxygen carriers—Oxygen release and reactivity with methane. Fuel 2011, 90, 941–950. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Optimization of spray dried attrition-resistant iron based oxygen carriers for chemical looping reforming. Chem. Eng. J. 2017, 309, 824–839. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Comparison of oxygen carriers for chemical-looping combustion. Therm. Sci. 2006, 10, 15. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Pérez-Astray, A.; Mendiara, T.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Chemical looping combustion of biomass: CLOU experiments with a Cu-Mn mixed oxide. Fuel Process. Technol. 2018, 172, 179–186. [Google Scholar] [CrossRef]
- Cabello, A.; Dueso, C.; García-Labiano, F.; Gayán, P.; Abad, A.; de Diego, L.F.; Adánez, J. Performance of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier with CH4 and H2S in a 500Wth CLC unit. Fuel 2014, 121, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Scala, F.; Chirone, R.; Salatino, P. Attrition phenomena relevant to fluidized bed combustion and gasification systems. In Fluidized Bed Technologies for Near-Zero Emission Combustion and Gasification; Elsevier: Amsterdam, The Netherlands, 2013; pp. 254–315. [Google Scholar]
- ASTM D5757-00, Standard Test Method for Determination of Attrition and Abrasion of Powdered Catalysts by Air Jets; ASTM International: West Conshohocken, PA, USA, 2000. [CrossRef]
- Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J.; Abad, A. Nickel−Copper Oxygen Carriers to Reach Zero CO and H 2 Emissions in Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2006, 45, 2617–2625. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Li, K.; Qing, S.; Zhu, X.; Wei, Y.; Li, Y.; Wang, H. Enhanced reducibility and redox stability of Fe2O3 in the presence of CeO2 nanoparticles. RSC Adv. 2014, 4, 47191–47199. [Google Scholar] [CrossRef]
- Jiang, B.; Li, L.; Bian, Z.; Li, Z.; Sun, Y.; Sun, Z.; Tang, D.; Kawi, S.; Dou, B.; Goula, M.A. Chemical looping glycerol reforming for hydrogen production by Ni@ZrO2 nanocomposite oxygen carriers. Int. J. Hydrogen Energy 2018, 43, 13200–13211. [Google Scholar] [CrossRef]
- Qin, L.; Cheng, Z.; Fan, J.A.; Kopechek, D.; Xu, D.; Deshpande, N.; Fan, L.-S. Nanostructure formation mechanism and ion diffusion in iron–titanium composite materials with chemical looping redox reactions. J. Mater. Chem. A 2015, 3, 11302–11312. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; Diniz da Costa, J.C. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.; Thursfield, A.; Metcalfe, I.S. A chemical looping process for hydrogen production using iron-containing perovskites. Energy Environ. Sci. 2011, 4, 4639. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M. Dense ceramic membranes for methane conversion. Catal. Today 2003, 82, 141–150. [Google Scholar] [CrossRef]
- Neal, L.M.; Shafiefarhood, A.; Li, F. Dynamic Methane Partial Oxidation Using a Fe2O3@La0.8Sr0.2FeO3−δ Core–Shell Redox Catalyst in the Absence of Gaseous Oxygen. ACS Catal. 2014, 4, 3560–3569. [Google Scholar] [CrossRef]
- Mihai, O.; Chen, D.; Holmen, A. Chemical looping methane partial oxidation: The effect of the crystal size and O content of LaFeO3. J. Catal. 2012, 293, 175–185. [Google Scholar] [CrossRef]
- Shafiefarhood, A.; Galinsky, N.; Huang, Y.; Chen, Y.; Li, F. Fe2O3@LaxSr1−xFeO3 Core-Shell Redox Catalyst for Methane Partial Oxidation. ChemCatChem 2014, 6, 790–799. [Google Scholar] [CrossRef]
- Martin, M. Materials in thermodynamic potential gradients. J. Chem. Thermodyn. 2003, 35, 1291–1308. [Google Scholar] [CrossRef] [Green Version]
- Schmalzried, H.; Laqua, W. Multicomponent oxides in oxygen potential gradients. Oxid. Met. 1981, 15, 339–353. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zheng, Y.; Chen, J.; Yu, B.; Chen, Y.; Liu, M. Controlling cation segregation in perovskite-based electrodes for high electro-catalytic activity and durability. Chem. Soc. Rev. 2017, 46, 6345–6378. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2-Fe2O3 complex oxides. Chem. Eng. J. 2010, 156, 512–518. [Google Scholar] [CrossRef]
- Koo, B.; Kim, K.; Kim, J.K.; Kwon, H.; Han, J.W.; Jung, W. Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists. Joule 2018, 2, 1476–1499. [Google Scholar] [CrossRef] [Green Version]
- Miya, K.; Otomo, J. Improvements in reaction kinetics and stability of ilmenite as oxygen carrier by surface modification with calcium titanate in redox cycles of chemical-looping systems. Chem. Eng. J. 2017, 327, 257–267. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Gorelov, V.P. High temperature electrical behavior of CaTi1−xFexO3−δ (x = 0–0.5). Oxygen-ion, electronic and proton conductivity. Solid State Ion. 2013, 253, 169–174. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Mattisson, T.; Chen, D.; Holmen, A.; Bjørgum, E. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1−xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4. Int. J. Greenh. Gas Control 2008, 2, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Neal, L.; Shafiefarhood, A.; Li, F. Effect of core and shell compositions on MeOx@LaySr1−yFeO3 core–shell redox catalysts for chemical looping reforming of methane. Appl. Energy 2015, 157, 391–398. [Google Scholar] [CrossRef]
- He, F.; Li, F. Perovskite promoted iron oxide for hybrid water-splitting and syngas generation with exceptional conversion. Energy Environ. Sci. 2015, 8, 535–539. [Google Scholar] [CrossRef]
- Cho, W.C.; Lee, J.K.; Nam, G.D.; Kim, C.H.; Cho, H.-S.; Joo, J.H. Degradation analysis of mixed ionic-electronic conductor-supported iron-oxide oxygen carriers for chemical-looping conversion of methane. Appl. Energy 2019, 239, 644–657. [Google Scholar] [CrossRef]
- Otomo, J.; Furumoto, Y.; Hatano, H.; Hatanaka, T.; Oshima, Y. Nickel oxide redox processes with oxide ion conductor-supported nickel oxide in dry and humidified methane: Effect of oxide ion conductors on induction period in nickel oxide reduction and subsequent hydrogen production. Fuel 2013, 104, 691–697. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kleitz, F.; Kaliaguine, S. Novel oxygen carriers for chemical looping combustion: La1−xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ. Sci. 2011, 4, 4258. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Leion, H.; Larring, Y.; Bakken, E.; Bredesen, R.; Mattisson, T.; Lyngfelt, A. Use of CaMn0.875Ti0.125O3 as Oxygen Carrier in Chemical-Looping with Oxygen Uncoupling. Energy Fuels 2009, 23, 5276–5283. [Google Scholar] [CrossRef]
- Isogai, S.; Kosaka, F.; Takimoto, I.; Hatano, H.; Oshima, Y.; Otomo, J. Acceleration of Fe2O3 Reduction Kinetics by Wet Methane with Calcium Titanate as Support. Chem. Lett. 2013, 42, 1438–1440. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Liu, S.; Huang, Z.; Wei, G.; Wang, G.; Cao, Y.; Zhao, K. La1−xSrxFeO3 perovskite-type oxides for chemical-looping steam methane reforming: Identification of the surface elements and redox cyclic performance. Int. J. Hydrogen Energy 2019, 44, 10265–10276. [Google Scholar] [CrossRef]
- Imanieh, M.H.; Rad, M.H.; Nadarajah, A.; González-Platas, J.; Rivera-López, F.; Martín, I.R. Novel perovskite ceramics for chemical looping combustion application. J. CO2 Util. 2016, 13, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.; Li, H.; Zhao, Z. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane. Int. J. Hydrogen Energy 2014, 39, 3243–3252. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Marin, G.B. Hydrogen Production from Methane and Carbon Dioxide by Catalyst-Assisted Chemical Looping. Top. Catal. 2011, 54, 907–913. [Google Scholar] [CrossRef]
- Hu, J.; Buelens, L.; Theofanidis, S.-A.; Galvita, V.V.; Poelman, H.; Marin, G.B. CO2 conversion to CO by auto-thermal catalyst-assisted chemical looping. J. CO2 Util. 2016, 16, 8–16. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Marin, G.B. Combined chemical looping for energy storage and conversion. J. Power Sources 2015, 286, 362–370. [Google Scholar] [CrossRef]
- Dharanipragada, N.V.R.A.; Galvita, V.V.; Poelman, H.; Buelens, L.C.; Detavernier, C.; Marin, G.B. Bifunctional Co- and Ni- ferrites for catalyst-assisted chemical looping with alcohols. Appl. Catal. B Environ. 2018, 222, 59–72. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Pressure-induced deactivation of core-shell nanomaterials for catalyst-assisted chemical looping. Appl. Catal. B Environ. 2019, 247, 86–99. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Sabbe, M.; Poelman, H.; Detavernier, C.; Marin, G.B. Controlling the stability of a Fe-Ni reforming catalyst: Structural organization of the active components. Appl. Catal. B Environ. 2017, 209, 405–416. [Google Scholar] [CrossRef]
- Dueso, C.; Izquierdo, M.T.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Gayán, P.; Adánez, J. Effect of H2S on the behaviour of an impregnated NiO-based oxygen-carrier for chemical-looping combustion (CLC). Appl. Catal. B Environ. 2012, 126, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.; Newby, R.; Keairns, D.; Woods, M. Oxygen Carrier Production Cost. In Proceedings of the 5th International conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018; p. 3. [Google Scholar]
- Pans, M.A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Abad, A.; Adánez, J. Performance of a low-cost iron ore as an oxygen carrier for Chemical Looping Combustion of gaseous fuels. Chem. Eng. Res. Des. 2015, 93, 736–746. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. On the use of a highly reactive iron ore in Chemical Looping Combustion of different coals. Fuel 2014, 126, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. Int. J. Greenh. Gas Control 2013, 19, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Adánez, J.; Gayán, P.; Adánez-Rubio, I.; Cuadrat, A.; Mendiara, T.; Abad, A.; García-Labiano, F.; de Diego, L.F. Use of Chemical-Looping processes for coal combustion with CO2 capture. Energy Procedia 2013, 37, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Schwebel, G.L.; Leion, H.; Krumm, W. Comparison of natural ilmenites as oxygen carriers in chemical-looping combustion and influence of water gas shift reaction on gas composition. Chem. Eng. Res. Des. 2012, 90, 1351–1360. [Google Scholar] [CrossRef]
- Linderholm, C.; Lyngfelt, A.; Cuadrat, A.; Jerndal, E. Chemical-looping combustion of solid fuels—Operation in a 10kW unit with two fuels, above-bed and in-bed fuel feed and two oxygen carriers, manganese ore and ilmenite. Fuel 2012, 102, 808–822. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Prompt considerations on the design of Chemical-Looping Combustion of coal from experimental tests. Fuel 2012, 97, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Cuadrat, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Relevance of the coal rank on the performance of the in situ gasification chemical-looping combustion. Chem. Eng. J. 2012, 195–196, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; Gayán, P.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Performance of a bauxite waste as oxygen-carrier for chemical-looping combustion using coal as fuel. Fuel Process. Technol. 2013, 109, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; García-Labiano, F.; Gayán, P.; Abad, A.; de Diego, L.F.; Adánez, J. Evaluation of the use of different coals in Chemical Looping Combustion using a bauxite waste as oxygen carrier. Fuel 2013, 106, 814–826. [Google Scholar] [CrossRef]
- Ortiz, M.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Abad, A.; Pans, M.A.; Adánez, J. Hydrogen production with CO2 capture by coupling steam reforming of methane and chemical-looping combustion: Use of an iron-based waste product as oxygen carrier burning a PSA tail gas. J. Power Sources 2011, 196, 4370–4381. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]



















| Institution | Location | Year | Capacity (kWth) | Ref |
|---|---|---|---|---|
| Vienna University of Technology | Vienna, Austria | 2009 | 120 | [43] |
| Hamburg University of Technology | Hamburg, Germany | 2012 | 25 | [44] |
| Chalmers University of Technology | Gothenburg, Sweden | 2012 | 100 | [45] |
| Darmstadt University of Technology | Darmstadt, Germany | 2012 | 1000 | [46] |
| Southeast University | Nanjing, China | 2012 | 50 | [47] |
| University of Utah | Salt Lake City, USA | 2012 | 200 | [17] |
| National Energy Technology Laboratory | Morgantown, USA | 2013 | 50 | [48] |
| Instituto de Carboquímica (ICB-CSIC) | Zaragoza, Spain | 2014 | 50 | [49] |
| Huazhong University of Sci. and Tech. | Wuhan, China | 2016 | 50 | [50] |
| VTT Technical Research Center | Espoo, Finland | 2016 | 50 | [51] |
| Japan Coal Energy Center | Tokyo, Japan | 2017 | 100 | [52] |
| Korean Institute of Energy Research | Daejeon, Korea | 2018 | 500 | [53] |
| Focus | Primary Fuel | Process | Reactor Type | |
|---|---|---|---|---|
| Combustion | Gas | CLC | Chemical Looping Combustion | f 1, m 2, p 3 |
| - | Solid | Syngas-CLC | Syngas-Chemical Looping Combustion | f, m, p |
| - | Solid | iG-CLC | In situ Gasification Chemical Looping Combustion | f, m |
| - | Solid | CLOU | Chemical Looping with Oxygen Uncoupling | f |
| - | Gas | GSC | Gas Switching Combustion | f |
| Chemicals | Gas | SR-CLR | Steam Reforming-Chemical Looping Reforming | f |
| - | Gas, liquid | a-CLR | Autothermal-Chemical Looping Reforming | f |
| - | Gas | GSR | Gas Switching Reforming | f |
| - | Gas | CSR | Chemical Switching Reforming | f |
| - | Gas, liquid | SE-CLSR | Sorption Enhanced-Chemical Looping Steam Reforming | f, m, p |
| - | Gas | CLHG/TRCH/OSD | Chemical Looping for Hydrogen Generation/Three Reactor Chemical Looping/One Step Decarbonization | f, m, p |
| - | Solid | SCL | Syngas Chemical Looping | m |
| - | Solid | CDCL | Coal Direct Chemical Looping | f, m |
| Oxygen Production | / | CLAS | Chemical Looping Air Separation | f, m, p |
| ADVANTAGES | DISADVANTAGES | |
|---|---|---|
| INTERCONNECTED FB | Flexible and continuous operation | Intraparticle diffusion resistance can reduce performance during continuous operation |
| Mature technology, easy to scale | Oxygen carrier attrition | |
| Stable production of reaction products | Fluidization problems by agglomeration | |
| Particle mixing reduces the risk for hot-spots | Stable solid circulation at higher pressures is more challenging | |
| High mass transfer rates | Fines separation at higher pressures is challenging | |
| Viable for solid, liquid, and gaseous fuels | ||
| PACKED BED | Ease of working under pressurized conditions | The risk for hot-spots when reactions are heavily exothermal |
| Inherent separation of gas and particles | Need for using a sophisticated gas switching system | |
| Negligible particle degradation | Possible deterioration due to particle volume changes | |
| More compact reactors, leading to reduced capital cost | Different reactions can occur when reaction front proceeds through the bed | |
| Batch operation | ||
| Not for solid fuels | ||
| ROTATING REACTOR | Continuous operation | Complex operation, difficult to scale |
| Ease of working under pressurized conditions | Reactor geometry depends on oxygen carrier and desired products | |
| Negligible particle degradation | High purge steam requirements lead to lower net efficiency | |
| More compact reactors, leading to reduced capital cost | Only for gaseous fuels | |
| No need for sophisticated gas switching systems | Possible deterioration due to particle volume changes | |
| The risk for hot-spots when reactions are heavily exothermal |
| Material | Synthesis | Support for | Key Points Related to Stability and Performance | Ref |
|---|---|---|---|---|
| La0.5Sr0.5Co0.5Fe0.5O3 | g.n. 1/s.d. 2 + f.g. 3 | Pure | No indication of decomposition after tests, suitable for CLC. | [280] |
| La0.6Sr0.4Co0.2Fe0.8O3−δ | c. 4 | Pure | The formation of La2CoO4 and sintering is observed. Steady hydrogen production. | [268] |
| LaFeO3−δ | g.n./s.d. + f.g. | Pure | Carbon formation at the end of the first reduction cycle. Earlier carbon formation in the following cycles. | [280] |
| La0.8Sr0.2FeO3−δ | g.n./s.d. + f.g. | Pure | Interesting for CLR, high selectivity for syngas generation. Carbon formation at the end of the first reduction cycle. | [280] |
| La0.5Sr0.5FeO3−δ | g.n./s.d. + f.g. | Pure | Interesting for CLR, high selectivity for syngas generation. No carbon formation during earlier reduction cycles. Increased Sr content decreases both selectivity towards H2 and reactivity with methane. | [280] |
| La0.7Sr0.3FeO3−δ | c. | Pure | No indication of structural changes during operation, no sintering is observed for 100 cycles. Steady hydrogen production. | [268] |
| La0.8Sr0.2FeO3−δ | s.s. 5 | 60 wt% Fe2O3 | Improved reactivity compared to TiO2, Al2O3, and YSZ-supported references. Also reactive at lower temperatures. Good resistance towards C-formation. | [250] |
| La0.7Sr0.3FeO3−δ | s.s. | Pure | Unstable under redox environment (reducing conditions). | [158] |
| La0.7Sr0.3FeO3−δ | s.s. | 50 wt% Fe2O3 | Better activity than CTF, more oxygen exchange, but less stable. | [158] |
| La0.8Sr0.2FeO3−δ | s.g. 6 | 50 mol% Fe2O3@LSF | Excellent reactivity and syngas selectivity. Fe-exsolution from LSF shell, reactivity controlled by O2- diffusion. | [270] |
| La0.8Sr0.2FeO3−δ | s.g. | 50 mol% Fe2O3 | Increased C deposition compared to the core-shell particle. A-site enrichment at the surface might lead to a core-shell particle after enough cycles. | [270] |
| La0.8Sr0.2FeO3−δ | s.s. | 60 wt% Fe2O3 | Good performance compared to OCs with inert supports. | [272] |
| La0.8Sr0.2FeO3−δ | s.g. | 50 wt% Fe2O3@LSF | The OC with nanoparticle cores is found to be 10–100 times more reactive for methane partial oxidation compared to the inert supported OCs. It also displays better reactivity, product selectivity, and resistance to C formation compared to composite. | [272] |
| La0.8Sr0.2FeO3−δ | s.g. | 50 mol% Co3O4@LSF | Exsolution of Co occurs with C deposition. Syngas selectivity is worse than Fe2O3-core. | [281] |
| La0.8Sr0.2FeO3−δ | s.g. | 50 mol% Fe2O3@LSF | Better syngas yield vs. LFO, SFO, and La0.5Sr0.5FeO3−δ. | [281] |
| La0.8Sr0.2FeO3−δ | s.g. | 50 mol% Mn2O3@LSF | Mn–Fe-spinel is formed, resulting in reduced OTC, good syngas selectivity, but low yields. | [281] |
| La0.7Sr0.3FeO3−δ | s.g. | 50 mol% Fe2O3@LSF | Better syngas yield vs. LFO, SFO, and La0.5Sr0.5FeO3−δ, deemed suitable for CLR. | [281] |
| La0.5Sr0.5FeO3−δ | s.g. | 50 mol% Fe2O3@LSF | SrFe12O19 formation. | [281] |
| SrFeO3 | s.g. | 50 mol% Fe2O3@SF | Stable in reducing environment, but poor performance due to complete avoidance of exsolution of Fe, SrFe12O19 formation. | [281] |
| LaFeO3 | s.g. | 50 mol% Fe2O3@LF | Extreme cokes formation during reduction due to extreme exsolution of Fe. | [281] |
| La0.8Sr0.2FeO3−δ | n.i. 7 | 60–75 wt% Fe2O3 | Good steam conversion due to reoxidation of exsolved Fe and reconstruction of decomposed LSF. | [282] |
| La0.8Sr0.2FeO3−δ | s.s. | 40 wt% Fe2O3 | Fractured into powder due to the redox environment. | [283] |
| La0.9Sr0.1AlO3−δ | s.s. + i. 8 | 35 wt% NiO | Ni-exsolution occurs (most likely). | [284] |
| LaCo0.83Fe0.1O3 | r.g. 9 | pure | Decomposition occurs as Co3O4 is detected after oxidation. | [285] |
| La0.81Ce0.09Co0.82Fe0.16O3 | r.g. | pure | Decomposition occurs as Co3O4 is detected after oxidation. Adding Ce does not improve reactivity. | [285] |
| LaMn0.73Fe0.38O3 | r.g. | pure | Decomposition occurs as Mn3O4 is detected after oxidation. | [285] |
| LaMnO3 | n.c. 10 | pure | Decomposition occurs as Mn3O4 is detected after oxidation. | [285] |
| CaxSr1−xTiyNi1−yO3−δ | s.g. | 30 mol% Fe2O3 | High initial activity. Slight deactivation due to the exchange of Ni in the lattice for Fe. Complete substitution results in stable redox activity. | [159] |
| Ce0.9Gd0.1O2−δ | s.s. | 35 wt% NiO | Ni-sintering occurs. | [284] |
| Ce0.8Gd0.2O2−δ | m.m. 11 | 30 wt% Fe2O3 | The reduction rate of iron oxide is clearly increased compared to ZrO2 and CeO2 supports. | [184] |
| Ce0.9Gd0.1O2−δ | s.s. | 40 wt% Fe2O3 | Formation of FeCeO3 and segregation of Fe from surface (exsolution). | [283] |
| Ce1−xFexO2−δ | c.p. 12 | x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 1 | The methane activation rate is dominated by surface iron dispersion, while the CO formation rate is related to oxygen mobility. During reduction, metallic Fe and CeFeO3 are formed, with the latter resulting in CeO2 and Fe during further reduction. Ion migration is observed, with Fe migrating towards the interior of the material. | [164] |
| CeO2 | c.p. | Fe2O3, ratio Ce:Fe 7:3 | High syngas selectivity due to dispersed Fe2O3 and Ce–Fe solid solution interaction. Reduced oxygen carriers could be reoxidized by air and restored in its initial state. Increased solid-solution of Fe in fluorite lattice after many redox cycles. | [276] |
| CeO2 | s.s., c.p. | 30 & 40 mol% Fe2O3 | High initial activity but notably deactivates with an increasing number of cycles. The inability of Fe to stabilize inside CeO2 support leads to largely segregated iron oxide on the surface, causing decreased accessibility of O2- to gaseous fuels. | [159] |
| CeO2 | m.m. | 30 wt% Fe2O3 | Lower reduction rate than GDC-supported material. Increased H2-reduction rate when FeO transforms to Fe (better electronic conductivity of CeO2 in its fully reduced state). | [184] |
| CeO2 | p. 13 + w.i. 14 | Ni (2.1 wt%, 8–11 wt% and 38.5 wt%) | Metallic Ni acts as a methane activator during dry reforming and remains in the metallic state due to the presence of CO2 under working conditions. Selectivity is practically complete towards syngas when oxidized by CO2. Indications of promotion of oxygen mobility from the support to active Ni-particles in the intermediate loading. Indications of differences in surface concentrations of all elements during operation. | [286] |
| CaTi0.125Mn0.875O3 | s.p.15/f.g. | Pure | Excellent results for CLOU. Ti incorporation in perovskite prevents coke formation. | [287] |
| CaTi(1-x)FexO3−δ | s.s. | 36 wt% Fe2O3 | Significant improvements in Fe2O3 reduction rate and lattice oxygen utilization using CTFO, likely induced by rapid ion transport at the interface between Fe2O3 and CTFO. | [288] |
| CaTi0.85Fe0.15O3−δ | s.s., s.g. | Pure, 50wt% Fe2O3 | Limited oxygen transfer capacity, better combining with transition metal as an active phase. More stable in comparison with LSF when together with the Fe-active phase. | [158] |
| CaTixFe1−xO3−δ | i. | surface modification of ilmenite | Calcination temperature influences the formation and location of CTFO in the ilmenite, resulting in different properties. Fe-exsolution in pure ilmenite shortens the lifetime in the OC material. In CTFO, Fe mobility is lower, leading to increased lifetimes. | [278] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. https://doi.org/10.3390/catal10080926
De Vos Y, Jacobs M, Van Der Voort P, Van Driessche I, Snijkers F, Verberckmoes A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts. 2020; 10(8):926. https://doi.org/10.3390/catal10080926
Chicago/Turabian StyleDe Vos, Yoran, Marijke Jacobs, Pascal Van Der Voort, Isabel Van Driessche, Frans Snijkers, and An Verberckmoes. 2020. "Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review" Catalysts 10, no. 8: 926. https://doi.org/10.3390/catal10080926