Rhodium (II)-Catalyzed Synthesis of Tetracyclic 3,4-Fused Indoles and Dihydroindoles
Abstract
:1. Introduction
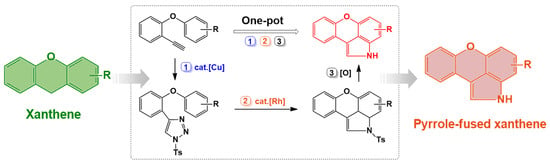
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Typical Procedure for the Synthesis of Triazole 4
3.3. Tetracycle 5 Synthesis by Rh(II) Catalyst
3.4. One-Pot Synthesis of 6 Starting from 1-ethynyl-2-phenoxybenzene
3.5. Synthesis of 9H-xanthen-9-one and Special Oxidative Aromatization Reaction of 4-(2-phenoxyphenyl)-1,2,3-triazole
3.6. Calculation of the Fluorescence Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lavis, L.D.; Rutkoski, T.J.; Raines, R.T. Tuning the pKa of Fluorescein to Optimize Binding Assays. Anal. Chem. 2007, 79, 6775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.Y.; Fan, K.Q.; Bai, Z.J.; Zhang, R.Q.; Zu, F.L.; Xu, J.X.; Li, X. Fluorescein applications as fluorescent probes for the detection of analytes. Trend. Anal. Chem. 2017, 97, 15. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavis, L.D. Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem. 2017, 86, 825. [Google Scholar] [CrossRef]
- Wang, L.; Du, W.; Hu, Z.; Uvdal, K.; Li, L.; Huang, W. Hybrid Rhodamine Fluorophores in the Visible/NIR Region for Biological Imaging. Angew. Chem. Int. Ed. 2019, 58, 14026. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142. [Google Scholar] [CrossRef] [Green Version]
- Kushida, Y.; Nagano, T.; Hanaoka, K. Silicon-substituted xanthene dyes and their applications in bioimaging. Analyst 2015, 140, 685. [Google Scholar] [CrossRef]
- Derayea, S.M.; Nagy, D.M. Application of a xanthene dye, eosin Y, as spectroscopic probe in chemical and pharmaceutical analysis; a review. Rev. Anal. Chem. 2018, 37, 20170020. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, Y.S.; Xu, W.N.; Zhao, Y.F.; Xie, H.Y.; Li, H.J.; Chen, X.F.; Zhao, R.S.; Guo, D.S. Far-red to near-infrared fluorescent probes based on silicon-substituted xanthene dyes for sensing and imaging. Trend. Anal. Chem. 2020, 122, 115704. [Google Scholar] [CrossRef]
- Zheng, H.; Zhan, X.Q.; Bian, Q.N.; Zhang, X.J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.G.; Berry, G.M.; Chen, C.H. Vita blue: A new 633-nm excitable fluorescent dye for cell analysis. Cytometry 1989, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.E.; Haugland, R.P.; Prendergast, F.G. Spectral and photophysical studies of benzo[c]xanthene dyes: Dual emission pH sensors. Anal. Biochem. 1991, 194, 330. [Google Scholar] [CrossRef]
- Liu, J.; Diwu, Z.; Leung, W.Y. Synthesis and photophysical properties of new fluorinated benzo[c]xanthene dyes as intracellular pH indicators. Bioorg. Med. Chem. Lett. 2001, 11, 2903. [Google Scholar] [CrossRef]
- Gu, J.; Du, W.; Chen, Y. Combined Asymmetric Aminocatalysis and Carbene Catalysis. Synthesis 2015, 47, 3451. [Google Scholar]
- Mahrwald, R. Organocatalytic methods for C-C bond formation. Drug Discov. Today 2013, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wen, M.; Zhang, S.W.; Pan, P.W.; Yu, X.X.; Deng, W.P. Unexpected O-H Insertion of Rhodium-Azavinylcarbenes with N-Acylhydrazones: Divergent Synthesis of 3,6-Disubstituted- and 3,5,6-Trisubstituted-1,2,4-Triazines. J. Org. Chem. 2017, 82, 1676. [Google Scholar] [CrossRef]
- Lavigne, F.; Kazzi, A.E.; Escudie, Y.; Maerten, E.; Kato, T.; Saffon-Merceron, N.; Branchadell, V.; Cossio, F.P.; Baceiredo, A. Azavinylidenephosphoranes: A class of cyclic push-pull carbenes. Chem. Eur. J. 2014, 20, 12528. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Dias, H.V.; Harlow, R.L. Electronic stabilization of nucleophilic carbenes. J. Am. Chem. Soc. 1992, 114, 5530. [Google Scholar] [CrossRef]
- Chuprakov, S.; Malik, J.A.; Zibinsky, M.; Fokin, V.V. Catalytic Asymmetric C-H Insertions of Rhodium(II) Azavinyl Carbenes. J. Am. Chem. Soc. 2011, 133, 10352. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Shi, Y.P.; Cheng, W.L.; Man, Z.M.; Yang, D.D.; Li, C.Y. Rhodium-Catalyzed Synthesis of 4-Bromo-1,2-dihydroisoquinolines: Access to Bromonium Ylides by the Intramolecular Reaction of a Benzyl Bromide and an α-Imino Carbene. Angew. Chem. Int. Ed. 2016, 55, 4557. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lei, X.Q.; Tang, Y.F. Rh(II)-catalyzed cycloadditions of 1-tosyl 1,2,3-triazoles with 2H-azirines: Switchable reactivity of Rh-azavinylcarbene as [2C]- or aza-[3C]-synthon. Chem. Commun. 2015, 51, 4507. [Google Scholar] [CrossRef] [PubMed]
- Stéphane, B.; Dagorne, S. Group 1 and 2 and Early Transition Metal Complexes Bearing N-Heterocyclic Carbene Ligands: Coordination Chemistry, Reactivity, and Applications. Chem. Rev. 2014, 114, 8747. [Google Scholar]
- Pal, K.; Hoque, A.; Volla, C.M.R. Rh-Catalyzed Denitrogenative Reaction of N-Sulfonyl-1,2,3-triazoles with Isatoic Anhydrides and Oxadiazolones. Chem. Eur. J. 2018, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Selander, N.; Worrell, B.T.; Fokin, V.V. Ring Expansion and Rearrangements of Rhodium(II) Azavinyl Carbenes. Angew. Chem. Int. Ed. 2012, 51, 13054. [Google Scholar] [CrossRef] [Green Version]
- Selander, N.; Worrell, B.T.; Chuprakov, S.; Velaparthi, S.; Fokin, V.V. Arylation of Rhodium(II) Azavinyl Carbenes with Boronic Acids. J. Am. Chem. Soc. 2012, 134, 14670. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.J.; Jeon, H.J.; Kim, J.H.; Kim, Y.; Lee, S. DMF as a Source of Oxygen and Aminomethine: Stereoselective 1,2-Insertion of Rhodium(II) Azavinyl Carbenes into the C=O Bond of Formamides for the Synthesis of cis-Diamino Enones. Org. Lett. 2014, 16, 2208. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Gevorgyan, V. Rh-catalyzed transannulation of N-tosyl-1,2,3-triazoles with terminal alkynes. Org. Lett. 2011, 13, 3746. [Google Scholar] [CrossRef] [Green Version]
- Zibinsky, M.; Fokin, V.V. Sulfonyl-1,2,3-Triazoles: Convenient Synthones for Heterocyclic Compounds. Angew. Chem. Int. Ed. 2013, 52, 1507. [Google Scholar] [CrossRef]
- Kubiak, R.W.; Mighion, J.D.; Wilkerson-Hill, S.M.; Alford, J.S.; Yoshidomi, T.; Davies, H.M.L. Enantioselective Intermolecular C-H Functionalization of Allylic and Benzylic sp3 C-H Bonds Using N-Sulfonyl-1,2,3-triazoles. Org. Lett. 2016, 18, 3118. [Google Scholar] [CrossRef] [Green Version]
- Alford, J.S.; Davies, H.M.L. Expanding the scope of donor/acceptor carbenes to N-phthalimido donor groups: Diastereoselective synthesis of 1-cyclopropane α-amino acids. Org. Lett. 2012, 14, 6020. [Google Scholar] [CrossRef]
- Khaidarov, A.R.; Rostovskii, N.V.; Zolotarev, A.A.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of 1-(2-Aminovinyl)indoles and 1,3’-Biindoles by Reaction of 2,2-Diaryl-Substituted 2H-Azirines with α-Imino Rh(II) Carbenoids. J. Org. Chem. 2019, 84, 3743. [Google Scholar] [CrossRef] [PubMed]
- Chuprakov, S.; Hwang, F.W.; Gevorgyan, V. Rh-catalyzed transannulation of pyridotriazoles with alkynes and nitriles. Angew. Chem. Int. Ed. 2007, 46, 4757. [Google Scholar] [CrossRef] [PubMed]
- Horneff, T.; Chuprakov, S.; Chernyak, N. Rhodium-Catalyzed Transannulation of 1,2,3-Triazoles with Nitriles. J. Am. Chem. Soc. 2008, 130, 14972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlets, Z.J.; Davies, H.M.L. Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles. Org. Lett. 2018, 20, 2168. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.H.; Jiang, P.; Jia, Z.H.; Xu, K.; Cao, J.; Chen, C.; Shen, M.H.; Xu, H.D. Expedient catalytic construction of azabicyclo[4.1.0]/[5.1.0] carbaldehydes via intramolecular cyclopropanation. Tetrahedron 2015, 71, 5124. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Fernandez, I.; Campo, T.M.; Palop, G.; Toledano-Pinedo, M.; Delgado-Martinez, P. Chemoselectivity Switching in the Rhodium-Catalyzed Reactions of 4-Substituted-1-sulfonyl-1,2,3-triazoles with Allenols: Noticeable Differences between 4-Acyl- and 4-Aryl-Triazoles. Adv. Synth. Catal. 2019, 361, 1160. [Google Scholar] [CrossRef]
- Bora, P.P.; Luo, Z.L.; Chen, L. Rh(II)-catalyzed intramolecular dearomatizing annulation of N-sulfonyl-1,2,3-triazoles: Synthesis of polycyclic spiroindolines. Tetrahedron 2016, 72, 1467. [Google Scholar] [CrossRef]
- Chuprakov, S.; Worrell, B.T.; Selander, N.; Sit, R.K.; Fokin, V.V. Stereoselective 1,3-Insertions of Rhodium(II) Azavinyl Carbenes. J. Am. Chem. Soc. 2014, 136, 195. [Google Scholar] [CrossRef] [Green Version]
- Grimster, N.; Zhang, L.; Fokin, V.V. Synthesis and Reactivity of Rhodium(II) N-Triflyl Azavinyl Carbenes. J. Am. Chem. Soc. 2010, 132, 2510. [Google Scholar] [CrossRef] [Green Version]
- Miura, T.; Biyajima, T.; Fujii, T.; Murakami, M. Synthesis of α-amino ketones from terminal alkynes via rhodium-catalyzed denitrogenative hydration of N-sulfonyl-1,2,3-triazoles. J. Am. Chem. Soc. 2012, 134, 194. [Google Scholar] [CrossRef]
- Jeon, H.J.; Jung, D.J.; Kim, J.H.; Kim, Y.; Bouffard, J.; Lee, S. From Triazoles to Imidazolines through the Sequential N-H Insertion of α-Imino Rhodium-Carbenes into β-Enamino Esters/Enamine-Imine Tautomerization/Conjugate Addition Cascade. J. Org. Chem. 2014, 79, 9865. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Funakoshi, Y.; Murakami, M. Intramolecular Dearomatizing [3+2] Annulation of α-Imino Carbenoids with Aryl Rings Furnishing 3,4-Fused Indole Skeletons. J. Am. Chem. Soc. 2014, 136, 2272. [Google Scholar] [CrossRef]
- Miura, T.; Zhao, Q.; Murakami, M. Selective Functionalization of Aromatic C(sp2)-H Bonds in the Presence of Benzylic C(sp3)-H Bonds by Electron-Deficient Carbenoids Generated from 4-Acyl-1-Sulfonyl-1,2,3-Triazoles. Angew. Chem. Int. Ed. 2017, 56, 16645. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Funakoshi, Y.; Fujimoto, Y.; Nakahashi, J.; Murakami, M. Facile Synthesis of 2,5-Disubstituted Thiazoles from Terminal Alkynes, Sulfonyl Azides, and Thionoesters. Org. Lett. 2015, 17, 2454. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Fujimoto, Y.; Funakoshi, Y.; Murakami, M. A Reaction of Triazoles with Thioesters to Produce β-Sulfanyl Enamides by Insertion of an Enamine Moiety into the Sulfur-Carbonyl Bond. Angew. Chem. Int. Ed. 2015, 54, 9967. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Davies, H.M.L. Scope of the Reactions of Indolyl- and Pyrrolyl-Tethered N-Sulfonyl-1,2,3-triazoles: Rhodium(II)-Catalyzed Synthesis of Indole- and Pyrrole-Fused Polycyclic Compounds. Org. Lett. 2017, 19, 1504. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson-Hill, S.M.; Haines, B.E.; Musaev, D.G.; Davies, H.M.L. Synthesis of [3a,7a]-Dihydroindoles by a Tandem Arene Cyclopropanation/3,5-Sigmatropic Rearrangement Reaction. J. Org. Chem. 2018, 83, 7939. [Google Scholar] [CrossRef]
- Xu, Y.P.; Hu, R.H.; Cai, M.Z. A facile synthesis of terminal arylacetylenes via Sonogashira coupling reactions catalyzed by MCM-41-supported mercapto palladium(0) complex. Chin. Chem. Lett. 2008, 19, 783. [Google Scholar] [CrossRef]
- Tanaka, K.; Yukimura, N.; Narasaka, K. Radical cyclization of O-pentafluorobenzoyloximes having a (cyclohexadiene)Fe(CO)3 moiety. Bull. Chem. Soc. Jpn. 2004, 77, 575. [Google Scholar] [CrossRef]
- Xu, H.D.; Pan, Y.P.; Ren, X.T.; Zhang, P.; Shen, M.H. A one-pot construction of acridones by rhodium catalyzed reaction of N-phenyl-2-(1-sulfonyl-1H-1,2,3-triazol-4-yl)aniline. Tetrahedron Lett. 2015, 56, 6734. [Google Scholar] [CrossRef]







| Comp. | Solvent | λex (nm) | λem (nm) | Quantum Yield (Φ) |
|---|---|---|---|---|
| 5a | CH2Cl2 | 388 | 443 | 0.31 |
| 6g | CH3CN | 370 | 471 | 0.45 |
| 6h | CH2Cl2 | 327 | 480 | 0.38 |
| 6j | DMSO | 436 | 552 | 0.52 |
| 7k | DMSO | 355 | 426 | 0.41 |
| 7l’ | DMSO | 355 | 457 | 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, H.; Bai, J.; Zhu, M.; Gao, J.; Anna; Zhang, S.; Li, C. Rhodium (II)-Catalyzed Synthesis of Tetracyclic 3,4-Fused Indoles and Dihydroindoles. Catalysts 2020, 10, 920. https://doi.org/10.3390/catal10080920
Qiao H, Bai J, Zhu M, Gao J, Anna, Zhang S, Li C. Rhodium (II)-Catalyzed Synthesis of Tetracyclic 3,4-Fused Indoles and Dihydroindoles. Catalysts. 2020; 10(8):920. https://doi.org/10.3390/catal10080920
Chicago/Turabian StyleQiao, Hongwei, Jiakun Bai, Mengyao Zhu, Juanhong Gao, Anna, Sichun Zhang, and Chao Li. 2020. "Rhodium (II)-Catalyzed Synthesis of Tetracyclic 3,4-Fused Indoles and Dihydroindoles" Catalysts 10, no. 8: 920. https://doi.org/10.3390/catal10080920