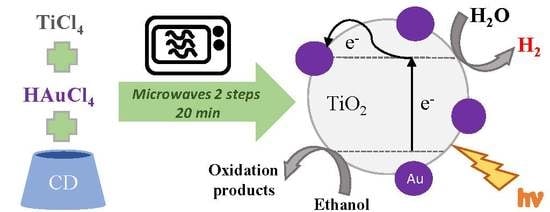
Fast Microwave Synthesis of Gold-Doped TiO2 Assisted by Modified Cyclodextrins for Photocatalytic Degradation of Dye and Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Au/TiO2 Materials with Cyclodextrins
3.3. Characterization Methods
3.3.1. Powder X-ray Diffraction
3.3.2. Nitrogen Adsorption-Desorption Isotherms
3.3.3. Diffuse Reflectance UV-Visible
3.3.4. Thermogravimetric Analysis (TGA) Coupled with Differential Scanning Calorimetry (DSC)
3.3.5. ICP Optical Emission Spectrometry
3.3.6. Transmission Electron Microscopy (TEM)
3.4. Photocatalytic Experiments
3.4.1. Photodegradation of Methyl Orange
3.4.2. Production of Hydrogen by Photoreduction of Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Youa, J.; Guoa, Y.; Guob, R.; Liub, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900–929. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV–vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B Environ. 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, D.; Liao, Y.; Li, F.; Zhang, H.; Xiang, Q. Constructing functionalized plasmonic gold/titanium dioxide nanosheets with small gold nanoparticles for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 555, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Iliev, V.; Tomova, D.; Bilyarska, L.; Tyuliev, G. Influence of the size of gold nanoparticles deposited on TiO2 upon the photocatalytic destruction of oxalic acid. J. Mol. Catal. A Chem. 2007, 263, 32–38. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, L.; Jia, H. Facile control of the self-assembly of gold nanoparticles by changing the capping agent structures. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 9–14. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Noël, S.; Léger, B.; Ponchel, A.; Philippot, K.; Denicourt-Nowicki, A.; Roucoux, A.; Monflier, E. Cyclodextrin-based systems for the stabilization of metallic(0) nanoparticles and their versatile applications in catalysis. Catal. Today 2014, 235, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, V.I.; Kumar, S.; Murthy, C.N. Cyclodextrin encapsulated monometallic and inverted core–shell bimetallic nanoparticles as efficient free radical scavengers. New J. Chem. 2016, 40, 1396–1402. [Google Scholar] [CrossRef]
- Aswathy, B.; Avadhani, G.S.; Suji, S.; Sony, G. Synthesis of β-cyclodextrin functionalized gold nanoparticles for the selective detection of Pb2+ ions from aqueous solution. Front. Mater. Sci. 2012, 6, 168–175. [Google Scholar] [CrossRef]
- Woo Chung, J.; Guo, Y.; Kwak, S.-Y.; Priestley, R.D. Understanding and controlling gold nanoparticle formation from a robust self-assembled cyclodextrin solid template. J. Mater. Chem. 2012, 22, 6017–6026. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Facile Synthesis and One-Dimensional Assembly of Cyclodextrin-Capped Gold Nanoparticles and Their Applications in Catalysis and Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Pande, S.; Ghosh, S.K.; Praharaj, S.; Panigrahi, S.; Basu, S.; Jana, S.; Pal, A.I.; Tsukuda, T.; Pal, T. Synthesis of Normal and Inverted Gold-Silver Core-Shell Architectures in α-Cyclodextrin and Their Applications in SERS. J. Phys. Chem. C 2007, 111, 10806–10813. [Google Scholar] [CrossRef]
- Zhu, H.; Goswami, N.; Yao, Q.; Chen, T.; Liu, Y.; Xu, Q.; Chen, D.; Lu, J.; Xie, J. Cyclodextrin–gold nanocluster decorated TiO2 enhances photocatalytic decomposition of organic pollutants. J. Mater. Chem. A 2018, 6, 1102–1108. [Google Scholar] [CrossRef]
- Fu, X.-C.; Zhang, C.; Li, X.-H.; Zhang, J.; Wei, G. Mono-6-thio-β-cyclodextrin-functionalized AuNP/two-dimensional TiO2 nanosheet nanocomposite for the electrochemical determination of trace methyl parathion in water. Anal. Methods 2019, 11, 4751–4760. [Google Scholar] [CrossRef]
- Lannoy, A.; Bleta, R.; Machut-Binkowski, C.; Addad, A.; Monflier, E.; Ponchel, A. Cyclodextrin-Directed Synthesis of Gold-Modified TiO2 Materials and Evaluation of Their Photocatalytic Activity in the Removal of a Pesticide from Water: Effect of Porosity and Particle Size. ACS Sustain. Chem. Eng. 2017, 5, 3623–3630. [Google Scholar] [CrossRef]
- Wen, P.; Wu, Z.; Han, Y.; Cravotto, G.; Wang, J.; Ye, B.-C. Microwave-Assisted Synthesis of a Novel Biochar-Based Slow-Release Nitrogen Fertilizer with Enhanced Water-Retention Capacity. ACS Sustain. Chem. Eng. 2017, 5, 7374–7382. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Berlier, G.; Caporaso, M.; Cravotto, G. Efficient Green Protocols for Preparation of Highly Functionalized β-Cyclodextrin-Grafted Silica. ACS Sustain. Chem. Eng. 2014, 2, 2595–2603. [Google Scholar] [CrossRef]
- Tabasso, S.; Calcio Gaudino, E.; Acciardo, E.; Manzoli, M.; Bonelli, B.; Cravotto, G. Microwave-Assisted Protocol for Green Functionalization of Thiophenes With a Pd/b-Cyclodextrin Cross-Linked Nanocatalyst. Front. Chem. 2020, 8, 253. [Google Scholar] [CrossRef]
- Stiufiuc, G.; Toma, V.; Moldovan, I.; Stiufiuc, R.; Lucaciu, C.M. One pot microwave assisted synthesis of cyclodextrins capped spherical gold nanoparticles. Dig. J. Nanomater. Biostruct. 2017, 12, 1089–1095. [Google Scholar]
- Tsuji, M. Microwave-Assisted Synthesis of Metallic Nanomaterials in Liquid Phase. ChemistrySelect 2017, 2, 805–819. [Google Scholar] [CrossRef]
- Qi, K.; Xin, J.H. Room-Temperature Synthesis of Single-Phase Anatase TiO2 by Aging and its Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2010, 2, 3479–3485. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martínez-Antonio, A. Synthesis of Gold Nanoparticles by Tetrachloroaurate Reduction with Cyclodextrins. Quim. Nova 2018, 41, 926–932. [Google Scholar]
- Liu, Y.; Male, K.B.; Bouvrette, P.; Luong, J.H.T. Control of the Size and Distribution of Gold Nanoparticles by Unmodified Cyclodextrins. Chem. Mater. 2003, 15, 4172–4180. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Zhu, H.; Zhu, Q.; Xia, Y. Three-in-One: Sensing; Self-Assembly and Cascade Catalysis of Cyclodextrin Modified Gold Nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef] [PubMed]
- Morawa Eblagon, K.; Pastrana-Martínez, L.M.; Pereira MF, R.; Figueiredo, J.L. Cascade conversion of cellobiose to gluconic acid: The large impact of the small modification of electronic interaction on the performance of Au/TiO2 bifunctional catalysts. Energy Technol. 2018, 6, 1675–1686. [Google Scholar] [CrossRef]
- Oros-Ruiza, S.; Zanellaa, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mitchell, D.R.G.; Prince, K.; Atanacio, A.J.; Caruso, R.A. Gold nanoparticle incorporation into porous titania networks using agarose gel templating technique for photocatlytic applications. Chem. Mater. 2008, 20, 3917–3926. [Google Scholar] [CrossRef]
- Willner, I.; Eichen, Y.; Willner, B. Supramolecular semiconductor receptor assemblies: Improved electron transfer at TiO2-β-cyclodextrin colloid interfaces. Res. Chem. Intermed. 1994, 20, 681–700. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Wang, Z.; Guo, Y.; Deng, N. Photocatalytic degradation of 4;4′-biphenol in TiO2 suspension in the presence of cyclodextrins: A trinity integrated mechanism. J. Mol. Catal. A Chem. 2009, 301, 134–139. [Google Scholar] [CrossRef]
- Lannoy, A.; Kania, N.; Bleta, R.; Fourmentin, S.; Machut-Binkowski, C.; Monflier, E.; Ponchel, A. Photocatalysis of Volatile Organic Compounds in water: Towards a deeper understanding of the role of cyclodextrins in the photodegradation of toluene over titanium dioxide. J. Colloid Interface Sci. 2016, 461, 317–325. [Google Scholar] [CrossRef]
- May-Masnou, A.; Soler, L.; Torras, M.; Salles, P.; Llorca, J.; Roig, A. Fast and Simple Microwave Synthesis of TiO2/Au Nanoparticles for Gas-Phase Photocatalytic Hydrogen Generation. Front. Chem. 2018, 10, 110. [Google Scholar] [CrossRef]
- Kanna, M.; Wognawa, S. Mixed amorphous and nanocrystalline TiO2 powders prepared by sol-gel method: Characterization and photocatalytic study. Mater. Chem. Phys. 2008, 110, 166–175. [Google Scholar] [CrossRef]
- Wang, X.; Caruso, R.A. Enhancing photocatalytic activity of titania materials by using porous structures and the addition of gold nanoparticles. J. Mater. Chem. 2011, 21, 20–28. [Google Scholar] [CrossRef]
- Dawson, A.; Kamat, P.V. Semiconductor-Metal Nanocomposites. Photoinduced Fusion and Photocatalysis of Gold-Capped TiO2 (TiO2/Gold) Nanoparticles. J. Phys. Chem. B 2001, 105, 960–966. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Malankowska, A.; Jarek, M.; Lisowski, W.; Nowaczyk, G.; Jurga, S.; Zaleska-Medynska, A. The effect of gold shape and size on the properties and visible light-induced photoactivity of Au-TiO2. Appl. Catal. B Environ. 2016, 196, 27–40. [Google Scholar] [CrossRef]
- Luna, A.L.; Matter, F.; Schreck, M.; Wohlwend, J.; Tervoort, E.; Colbeau-Justin, C.; Niederberger, M. Monolithic metal-containing TiO2 aerogels assembled from crystalline pre-formed nanoparticles as efficient photocatalysts for H2 generation. Appl. Catal. B Environ. 2020, 267, 118660. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for solar hydrogen conversion. J. Mater. 2017, 3, 96–111. [Google Scholar]
- Wang, X.; Tian, J.; Fei, C.; Lv, L.; Wang, Y.; Cao, G. Rapid construction of TiO2 aggregates using microwave assisted synthesis and its application for dye-sensitized solar cells. RSC Adv. 2015, 5, 8622–8629. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Li, J. β-Cyclodextrin controlled assembling nanostructures from gold nanoparticles to gold nanowires. Chem. Phys. Lett. 2004, 389, 14–18. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Abstracts of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCR, Toulouse, France; International Union of Crystallography: Chester, UK, 1990; p. 127. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. In Materials Science Forum, Proceedings of the 7th European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2000; Delhez, R., Mittenmeijer, E.J., Eds.; Trans Tech Publications: Zurich, Switzerland, 2000; pp. 118–123. [Google Scholar]
- Khore, S.K.; Kadam, S.R.; Naik, S.D.; Kale, B.B.; Sonawane, R.S. Solar light active plasmonic Au@TiO2 nanocomposite with superior photocatalytic performance for H2 production and pollutant degradation. New J. Chem. 2018, 42, 10958–10968. [Google Scholar] [CrossRef]








| Sample | SBET (m2.g−1) a | Pore Volume (cm3. g−1) b | Pore Size (nm) c | Au Content (wt %) d |
|---|---|---|---|---|
| TiO2-control | 240 | 0.26 | 4.2 | - |
| TiO2@Au | 230 | 0.30 | 4.8 | 2.3 |
| TiO2@Au-RB | 230 | 0.22 | 4.0 | 2.0 |
| TiO2@Au-HP | 260 | 0.30 | 4.8 | 2.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machut, C.; Kania, N.; Léger, B.; Wyrwalski, F.; Noël, S.; Addad, A.; Monflier, E.; Ponchel, A. Fast Microwave Synthesis of Gold-Doped TiO2 Assisted by Modified Cyclodextrins for Photocatalytic Degradation of Dye and Hydrogen Production. Catalysts 2020, 10, 801. https://doi.org/10.3390/catal10070801
Machut C, Kania N, Léger B, Wyrwalski F, Noël S, Addad A, Monflier E, Ponchel A. Fast Microwave Synthesis of Gold-Doped TiO2 Assisted by Modified Cyclodextrins for Photocatalytic Degradation of Dye and Hydrogen Production. Catalysts. 2020; 10(7):801. https://doi.org/10.3390/catal10070801
Chicago/Turabian StyleMachut, Cécile, Nicolas Kania, Bastien Léger, Frédéric Wyrwalski, Sébastien Noël, Ahmed Addad, Eric Monflier, and Anne Ponchel. 2020. "Fast Microwave Synthesis of Gold-Doped TiO2 Assisted by Modified Cyclodextrins for Photocatalytic Degradation of Dye and Hydrogen Production" Catalysts 10, no. 7: 801. https://doi.org/10.3390/catal10070801