Enhanced Photodegradation of Synthetic Dyes Mediated by Ag3PO4-Based Semiconductors under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Photocatalytic Activity
2.3. Formation of Reactive Oxygen Species
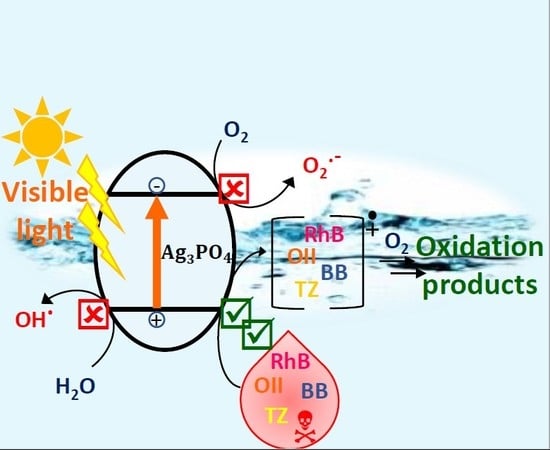
2.4. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Photocatalysts
3.3. Scanning Electron Microscopy Analysis
3.4. Photophysical Experiments
3.5. Photocatalytic Degradation of Dyes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Gagol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation-A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced Oxidation Processes for the Removal of Food Dyes in Wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Fernandez, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trend Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Gregory, P. Azo dyes-Structure carcinogenicity relationships. Dye. Pigment. 1986, 7, 45–56. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Ismael, M.; Elhaddad, E.; Taffa, D.H.; Wark, M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts 2017, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Ismael, M.; Wark, M. Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Withers, R.L. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Jinfeng, Z.; Tao, Z. Preparation and characterization of highly efficient and stable visible-light-responsive photocatalyst AgBr/Ag3PO4. J. Nanomater. 2013, 2013, 565074. [Google Scholar] [CrossRef] [Green Version]
- Wardman, P. Reduction potentials of one-electron couples involving free-radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Pitre, S.P.; McTiernan, C.D.; Scaiano, J.C. Understanding the Kinetics and Spectroscopy of Photoredox Catalysis and Transition-Metal-Free Alternatives. Acc. Chem. Res. 2016, 49, 1320–1330. [Google Scholar] [CrossRef]
- Cao, J.; Luo, B.; Lin, H.; Xu, B.; Chen, S. Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methyl orange. J. Hazard. Mater. 2012, 217–218, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhu, N.; Zhao, Y.; Li, J.; Liu, L. Sunlight-Assisted Degradation of Dye Pollutants in Ag3PO4 Suspension. Ind. Eng. Chem. Res. 2012, 51, 5167–5173. [Google Scholar] [CrossRef]
- Ge, M. Photodegradation of rhodamine B and methyl orange by Ag3PO4 catalyst under visible light irradiation. Chin. J. Catal. 2014, 35, 1410–1417. [Google Scholar] [CrossRef]
- Qamar, M.; Elsayed, R.B.; Alhooshani, K.R.; Ahmed, M.I.; Bahnemann, D.W. Chemoselective and highly efficient conversion of aromatic alcohols into aldehydes photo-catalyzed by Ag3PO4 in aqueous suspension under simulated sunlight. Catal. Commun. 2015, 58, 34–39. [Google Scholar] [CrossRef]
- Taheri, M.E.; Petala, A.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Fast photocatalytic degradation of bisphenol A by Ag3PO4/TiO2 composites under solar radiation. Catal. Today 2017, 280, 99–107. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Chen, M.; Zeng, G.; Wang, D.; Chen, F.; Tan, X. Application of silver phosphate-based photocatalysts: Barriers and solutions. Chem. Eng. J. 2019, 366, 339–357. [Google Scholar] [CrossRef]
- Zwara, J.; Grabowska, E.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Shape-dependent enhanced photocatalytic effect under visible light of Ag3PO4 particles. J. Photochem. Photobiol. A 2018, 367, 240–252. [Google Scholar] [CrossRef]
- Petala, A.; Spyrou, D.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Immobilized Ag3PO4 photocatalyst for micro-pollutants removal in a continuous flow annular photoreactor. Catal. Today 2019, 328, 223–229. [Google Scholar] [CrossRef]
- Cruz-Filho, J.F.; Costa, T.M.S.; Lima, M.S.; Silva, L.J.; Santos, R.S.; Cavalcante, L.S.; Luz, G.E. Effect of different synthesis methods on the morphology, optical behavior, and superior photocatalytic performances of Ag3PO4 sub-microcrystals using white-light-emitting diodes. J. Photochem. Photobiol. A 2019, 377, 14–25. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Y.; Yang, Y.; Chen, Y.; Yang, Z.; Wang, P.; Feng, W. Influence of operational parameters on photocatalytic decolorization of a cationic azo dye under visible-light in aqueous Ag3PO4. Inorg. Chem. Commun. 2020, 115, 107850. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Gul, H.; Azam, M.; Lee, J.; Vikrant, K.; Kim, K.-H. Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. J. Clean. Prod. 2020, 254, 120031. [Google Scholar] [CrossRef]
- Tab, A.; Bellal, B.; Belabed, C.; Dahmane, M.; Trari, M. Visible light assisted photocatalytic degradation and mineralization of Rhodamine B in aqueous solution by Ag3PO4. Optik 2020, 214, 164858. [Google Scholar] [CrossRef]
- Hamrouni, A.; Azzouzi, H.; Rayes, A.; Palmisano, L.; Ceccato, R.; Parrino, F. Enhanced Solar Light Photocatalytic Activity of Ag Doped TiO2-Ag3PO4 Composites. Nanomaterials 2020, 10, 795. [Google Scholar] [CrossRef] [Green Version]
- Rawal, S.B.; Sung, S.D.; Lee, W.I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation. Catal. Commun. 2012, 17, 131–135. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Zhang, T.; Li, D. Synthesis and characterization of Ag3PO4 immobilized in bentonite for the sunlight-driven degradation of Orange II. Appl. Catal. B 2013, 134–135, 1–6. [Google Scholar] [CrossRef]
- Molla, M.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Knaeco, S. Evaluation of Reaction Mechanism for Photocatalytic Degradation of Dye with Self-Sensitized TiO2 under Visible Light Irradiation. Open J. Inorg. Non-Met. Mater. 2017, 7, 1–7. [Google Scholar]
- Trasatti, S. The absolute electrode potential: An explanatory note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955. [Google Scholar] [CrossRef]
- Ahmad, I.; Murtaza, S.; Ahmed, S. Electrochemical and photovoltaic study of sunset yellow and tartrazine dyes. Mon. Chem. 2015, 146, 1631–1640. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous voltammetric determination of Brilliant Blue and Tartrazine in real samples at the surface of a multi-walled carbon nanotube paste electrode. Anal. Methods 2011, 3, 2842–2847. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinodgopal, K.; Wynkoop, D.E.; Kamat, P.V. Environmental Photochemistry on Semiconductor Surfaces: Photosensitized Degradation of a Textile Azo Dye, Acid Orange 7, on TiO2 Particles Using Visible Light. Environ. Sci. Technol. 1996, 30, 1660–1666. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Photochemistry of textile azo dyes. Spectral characterization of excited state, reduced and oxidized forms of Acid Orange 7. J. Photochem. Photobiol. A 1994, 83, 141–146. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Chebotarev, A.N.; Bevziuk, K.V.; Snigur, D.V.; Bazel, Y.R. The brilliant blue FCF ion-molecular forms in solutions according to the spectrophotometry data. Russ. J. Phys. Chem. A 2017, 91, 1907–1912. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic Decolorization of Dye with Self-Dye-Sensitization under Fluorescent Light Irradiation. ChemEngineering 2017, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Baiocchi, C.; Brussino, M.C.; Pramauro, E.; Prevot, A.B.; Palmisano, L.; Marci, G. Characterization of methyl orange and its photocatalytic degradation products by HPLC/UV–VIS diode array and atmospheric pressure ionization quadrupole ion trap mass spectrometry. Int. J. Mass Spectrom. 2002, 214, 247–256. [Google Scholar] [CrossRef]
- Liu, R.; Hu, P.; Chen, S. Photocatalytic activity of Ag3PO4 nanoparticle/TiO2 nanobelt heterostructures. Appl. Surf. Sci. 2012, 258, 9805–9809. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Liu, Z.; Chen, J.; Wu, Y.; Guo, M.; Na, P. In-situ deposition of Ag3PO4 on TiO2 nanosheets dominated by (001) facets for enhanced photocatalytic activities and recyclability. Ceram. Int. 2017, 43, 11588–11595. [Google Scholar] [CrossRef]
- Kim, W.J.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption/photocatalytic activity and fundamental natures of BiOCl and BiOClxI1−x prepared in water and ethylene glycol environments, and Ag and Au-doping effects. Appl. Catal. B 2014, 147, 711–725. [Google Scholar] [CrossRef]
- Cao, J.; Luo, B.; Lin, H.; Chen, S. Synthesis, characterization and photocatalytic activity of AgBr/H2WO4 composite photocatalyst. J. Mol. Catal. A Chem. 2011, 344, 138–144. [Google Scholar] [CrossRef]
- Ishibashi, K.-i.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Xiao, Q.; Si, Z.; Zhang, J.; Xiao, C.; Tan, X. Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J. Hazard. Mat. 2008, 150, 62–67. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Reszka, K.; Chignell, C.F. Spectroscopic studies of cutaneous photosensitizing agents IV. The photolysis of benoxaprofen, an anti-inflammatory drug with phototoxic properties. Photochem. Photobiol. 1983, 38, 281–291. [Google Scholar] [CrossRef]
- Burns, J.M. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, S.; Song, W. Photochemically Induced Formation of Reactive Oxygen Species (ROS) from Effluent Organic Matter. Environ. Sci. Technol. 2014, 48, 12645–12653. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D, N.; Kondamareddy, K.K.; Bin, H.; Lu, D.; Kumar, P.; Dwivedi, R.K.; Fu, D. Enhanced visible light photodegradation activity of RhB/MB from aqueous solution using nanosized novel Fe-Cd co-modified ZnO. Sci. Rep. 2018, 8, 10691. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ouyang, S.; Cao, J.; Ye, J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef] [PubMed]










| Dye | Max (nm) | (M−1 cm−1) | Eox (dye+/dye, V vs. NHE) | E (HOMO–LUMO Gap, eV) | Eox * (dye+/1dye *, V vs. NHE) |
|---|---|---|---|---|---|
| Tartrazine (TZ) | 429.5 [41] | 20,810 [41] | +1.25 [42] | 2.33 | −1.08 [41] |
| Orange II (OII) | 480 [43] | 15,400 [43] | +0.76 [44] | 2.36 [45] | −1.60 [45] |
| Rhodamine B (RhB) | 545 [43] | 106,000 [43] | +1.15 [46] | 2.22 [46] | −1.07 [46] |
| Brilliant Blue FCF (BB) | 625 [47] | 97,000 [47] | +1.06 [42] | 1.86 | −0.80 |
| Photocatalyst | TZ | OII | RhB | BB |
|---|---|---|---|---|
| Ag3PO4 cubes | 6.9 × 10−3 | 2.0 × 10−1 | 8.1 × 10−3 | 8.8 × 10−3 |
| Ag3PO4 rhombic dodecahedrons | 1.8 × 10−3 | 8.5 × 10−2 | 6.1 × 10−3 | 5.4 × 10−4 |
| Ag3PO4 spheres | 1.6 × 10−2 | 1.3 × 10−1 | 5.4 × 10−3 | 5.1 × 10−3 |
| TiO2–Ag3PO4 | 1.8 × 10−2 | 2.0 × 10−1 | 4.4 × 10−3 | 4.0 × 10−3 |
| TiO2 | 1.9 × 10−2 | 1.8 × 10−2 | 1.9 × 10−2 | 1.3 × 10−2 |
| Photocatalyst | TZ | OII | RhB | BB |
|---|---|---|---|---|
| Ag3PO4 cubes | 3.5 × 10−2 | 4.1 × 10−1 | 4.8 × 10−2 | 1.2 × 10−2 |
| Ag3PO4 rhombic dodecahedrons | 4.3 × 10−2 | 2.8 × 10−1 | 2.5 × 10−2 | 1.7 × 10−2 |
| Ag3PO4 spheres | 4.4 × 10−2 | 3.2 × 10−1 | 5.6 × 10−2 | 1.0 × 10−2 |
| TiO2–Ag3PO4 | 3.4 × 10−2 | 3.0 × 10−1 | 4.4 × 10−2 | 1.6 × 10−2 |
| TiO2 | 5.7 × 10−4 | 6.7 × 10−4 | 4.7 × 10−3 | 8.1 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavanello, A.; Blasco, A.; Johnston, P.F.; Miranda, M.A.; Marin, M.L. Enhanced Photodegradation of Synthetic Dyes Mediated by Ag3PO4-Based Semiconductors under Visible Light Irradiation. Catalysts 2020, 10, 774. https://doi.org/10.3390/catal10070774
Pavanello A, Blasco A, Johnston PF, Miranda MA, Marin ML. Enhanced Photodegradation of Synthetic Dyes Mediated by Ag3PO4-Based Semiconductors under Visible Light Irradiation. Catalysts. 2020; 10(7):774. https://doi.org/10.3390/catal10070774
Chicago/Turabian StylePavanello, Alice, Alejandro Blasco, Peter F. Johnston, Miguel A. Miranda, and Maria Luisa Marin. 2020. "Enhanced Photodegradation of Synthetic Dyes Mediated by Ag3PO4-Based Semiconductors under Visible Light Irradiation" Catalysts 10, no. 7: 774. https://doi.org/10.3390/catal10070774