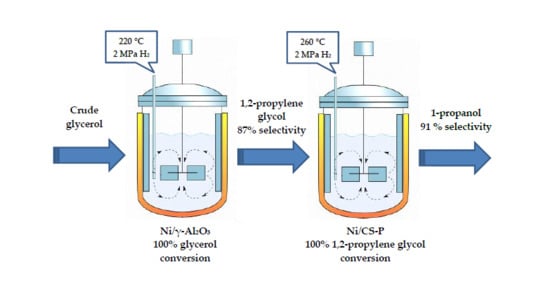
High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Supports and Catalysts
2.2. Catalytic Activity
3. Materials and Methods
3.1. Synthesis of Supports and Catalysts
3.2. Characterization of Supports and Catalysts
3.3. Crude Glycerol Characterization
3.4. Catalytic Actitivy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chheda, J.N.; Hubert, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Edit. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Beltrami, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, N.; Nordin, N.M.; Nadzri, A.H.A.; Nik, Y.A.; Kassim, M.B.; Yarmo, M.A. Enhanced activity of Ru/TiO2 catalyst using bisupport, bentonite-TiO2 for hydrogenolysis of glycerol in aqueous media. Appl. Catal. A Gen. 2012, 419, 133–141. [Google Scholar] [CrossRef]
- Salazar, J.B.; Falcone, D.D.; Pham, H.N.; Datye, A.K.; Passos, F.B.; Davis, R.J. Selective production of 1,2-propanediol by hydrogenolysis of glycerol over bimetallic Ru–Cu nanoparticles supported on TiO2. Appl. Catal. A Gen. 2014, 482, 137–144. [Google Scholar] [CrossRef]
- Lopez, A.; Aragón, J.A.; Hernández-Cortez, J.G.; Mosqueira, M.L.; Martínez-Palou, R. Study of hydrotalcite-supported transition metals as catalysts for crude glycerol hydrogenolysis. Mol. Catal. 2019, 468, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Xiang, M.; Wu, D. Hydrogenolysis of glycerol over bimetallic Cu-Ni catalysts supported on hierarchically porous SAPO-11 zeolite. Catal. Commun. 2019, 119, 170–175. [Google Scholar] [CrossRef]
- Grabysch, T.; Muhler, M.; Peng, B.X. The kinetics of glycerol hydrodeoxygenation to 1,2-propanediol over Cu/ZrO2 in the aqueous phase. Appl. Catal. A Gen. 2019, 576, 47–53. [Google Scholar] [CrossRef]
- Varghese, J.J.; Cao, L.; Robertson, C.; Yang, Y.; Gladden, L.F.; Lapkin, A.A.; Mushrif, S.H. Synergistic Contribution of the Acidic Metal Oxide–Metal Couple and Solvent Environment in the Selective Hydrogenolysis of Glycerol: A Combined Experimental and Computational Study Using ReOx-Ir as the Catalyst. ACS Catal. 2019, 9, 485–503. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Q.; Zhu, M.; Zhao, Y.; Liu, Y.; Zhou, C.; Yang, Y.; Cao, Y. Interface synergy between IrOx and H-ZSM-5 in selective C–O hydrogenolysis of glycerol toward 1,3-propanediol. J. Catal. 2019, 375, 339–350. [Google Scholar] [CrossRef]
- Liu, L.; Kawakami, S.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Highly active iridium-rhenium catalyst condensed on silica support for hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2019, 256, 117775. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Periasamy, S.; Chary, K.V.R. High Efficiency Conversion of Glycerol to 1,3-Propanediol Using a Novel Platinum–Tungsten Catalyst Supported on SBA-15. Ind. Eng. Chem. Res. 2015, 498, 88–98. [Google Scholar] [CrossRef]
- Bhanuchander, P.; Priya, S.S.; Kumar, V.P.; Hussain, S.; Rajan, N.P.; Bhargava, S.K.; Chary, K.V.R. Direct Hydrogenolysis of Glycerol to Biopropanols over Metal Phosphate Supported Platinum Catalysts. Catal. Lett. 2017, 147, 845–855. [Google Scholar] [CrossRef]
- Li, C.; He, B.; Ling, Y.; Tsang, C.-W.; Liang, C. Glycerol hydrogenolysis to n-propanol over Zr-Al composite oxide-supported Pt catalysts. Chin. J. Catal. 2018, 39, 1121–1128. [Google Scholar] [CrossRef]
- Marinas, A.; Bruijnincx, P.; Ftouni, J.; Urbano, F.J.; Pinel, C. Sustainability metrics for a fossil- and renewable-based route for 1,2-propanediol production: A comparison. Catal. Today 2015, 239, 31–37. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Zheng, H.; Mo, T.; Li, Y. One-step hydrogenolysis of glycerol to biopropanols over Pt-H4SiW12O40/ZrO2 catalysts. Green Chem. 2012, 14, 2607–2616. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C + an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Van Ryneveld, E.; Mahomed, A.S.; Van Heerden, P.S.; Friedrich, H.B. Direct Hydrogenolysis of Highly Concentrated Glycerol Solutions Over Supported Ru, Pd and Pt Catalyst Systems. Catal. Lett. 2011, 141, 958–967. [Google Scholar] [CrossRef]
- Van Ryneveld, E.; Mahomed, A.S.; Van Heerden, P.S.; Green, M.J.; Friedrich, H.B. A catalytic route to lower alcohols from glycerol using Ni-supported catalysts. Green Chem. 2011, 13, 1819–1827. [Google Scholar] [CrossRef]
- Mai, C.T.Q.; Ng, F.T.T. Effect of Metals on the Hydrogenolysis of Glycerol to Higher Value Sustainable and Green Chemicals Using a Supported HSiW Catalyst. Org. Process. Res. Dev. 2016, 20, 1774–1780. [Google Scholar] [CrossRef]
- Gallegos-Suarez, E.; Pérez-Cadenas, M.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I.; Arcoya, A. Effect of the functional groups of carbon on the surface and catalytic properties of Ru/C catalysts for hydrogenolysis of glycerol. Appl. Surf. Sci. 2013, 287, 108–116. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Xie, Y.; Wu, X.; Chen, C.; Ma, W.; Dong, Q.; Hou, Z. Catalytic transformation of glycerol to 1-propanol by combining zirconium phosphate and supported Ru catalysts. RSC Adv. 2016, 6, 29769–29778. [Google Scholar] [CrossRef]
- Delgado, S.N.; Yap, D.; Vivier, L.; Especel, C. Influence of the nature of the support on the catalytic properties of Pt-based catalysts for hydrogenolysis of glycerol. J. Mol. Catal. A Chem. 2013, 367, 89–98. [Google Scholar] [CrossRef]
- Zhu, S.; Qiuc, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids. Catal. Today 2013, 212, 120–126. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Glycerol Hydrogenolysis Promoted by Supported Palladium Catalysts. ChemSusChem 2011, 4, 1143–1150. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Ma, L.; He, D. Influence of Pd precursors and Cl addition on performance of Pd-Re catalysts in glycerol hydrogenolysis to propanediols. Appl. Catal. A Gen. 2016, 522, 13–20. [Google Scholar] [CrossRef]
- Samudrala, S.P.; Bhattacharya, S. Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts. Catalysts 2018, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Shozi, M.L.; Dasireddy, V.D.B.C.; Singh, S.; Govender, A.; Mohlala, P.; Friedrich, H.B. The effect of rhenium on the conversion of glycerol to mono-alcohols over nickel catalysts under continuous flow conditions. Sustain. Energy Fuels 2019, 3, 2038–2047. [Google Scholar] [CrossRef]
- Shozi, M.L.; Dasireddy, V.D.B.C.; Singh, S.; Mohlala, P.; Morgan, D.J.; Friedrich, H.B. Hydrogenolysis of Glycerol to Monoalcohols over Supported Mo and W Catalysts. ACS Sustain. Chem. Eng. 2016, 4, 5752–5760. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Lv, Y.; Xi, Y.; Qu, Y.; Phillips, D.L.; Liu, C. Hydrogenolysis of Glycerol by the Combined Use of Zeolite and Ni/Al2O3 as Catalysts: A Route for Achieving High Selectivity to 1-Propanol. Energy Fuels 2014, 28, 3345–3351. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N.; Sai Prasad, P.S.; Lingaiah, N. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A Gen. 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. Quantifying the dominant factors in Cu catalyst deactivation during glycerol hydrogenolysis. J. Ind. Eng. Chem. 2017, 54, 270–277. [Google Scholar] [CrossRef]
- Balaraju, M.; Jagadeeswaraiah, K.; Sai Prasad, P.S.; Lingaiah, N. Catalytic hydrogenolysis of biodiesel derived glycerol to 1,2-propanediol over Cu-MgO catalysts. Catal. Sci. Technol. 2012, 2, 1967–1976. [Google Scholar] [CrossRef]
- Dam, J.T.; Kapteijn, F.; Djanashvili, K.; Hanefeld, U. Tuning selectivity of Pt/CaCO3 in glycerol hydrogenolysis-A Design of Experiments approach. Catal. Commun. 2011, 13, 1–5. [Google Scholar] [CrossRef]
- Gatti, M.N.; Mizrahi, M.D.; Ramallo-Lopez, J.M.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Improvement of the catalytic activity of Ni/SiO2-C by the modification of the support and Zn addition: Bio-propylene glycol from glycerol. Appl. Catal. A Gen. 2017, 548, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Bio-propylene glycol by liquid phase hydrogenolysis of glycerol with Ni/SiO2-C catalysts. Catal. Today 2017, 296, 26–34. [Google Scholar] [CrossRef]
- Snaz, J.; Campelo, J.M.; Marinas, J.M. NMR characterization of synthetic and modified aluminum orthophosphates. J. Catal. 1991, 130, 642–652. [Google Scholar] [CrossRef]
- Campelo, J.M.; Jaraba, M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Effect of Phosphate Precursor and Organic Additives on the Structural and Catalytic Properties of Amorphous Mesoporous AlPO4 Materials. Chem. Mater. 2003, 15, 3352–3364. [Google Scholar] [CrossRef]
- Raddi de Araujo, L.R.; Scofield, C.F.; Pastura, N.M.R.; Gonzalez, W.A. H3PO4/Al2O3 Catalysts: Characterization and Catalytic Evaluation of Oleic Acid Conversion to Biofuels and Biolubricant. Mater. Res. 2006, 9, 181–184. [Google Scholar] [CrossRef]
- Gervasini, A.; Fenyvesi, J.; Auroux, A. Study of the acidic character of modified metal oxide surfaces using the test of isopropanol decomposition. Catal. Lett. 1997, 43, 219–228. [Google Scholar] [CrossRef]
- Moffat, J.B.; Vetrivel, R.; Viswanathan, B. A model cluster study of the acid-base properties of phosphate catalysts. J. Mol. Catal. 1985, 30, 171–180. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; Pizarro, P.; De la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Influence of the Ni/P ratio and metal loading on the performance of NixPy/SBA-15 catalysts for the hydrodeoxygenation of methyl oleate. Fuel 2015, 144, 60–70. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Jiménez-López, A. A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene. J. Catal. 2009, 263, 4–15. [Google Scholar] [CrossRef]
- Senseni, A.Z.; Meshkani, F.; Rezaei, M. Steam reforming of glycerol on mesoporous nanocrystalline Ni/Al2O3 catalysts for H2 production. Int. J. Hydrogen Energy 2016, 41, 20137–20146. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Li, S.; Han, Z.; Wang, T.; Ma, X.; Gong, J. Hydrogen Production via Glycerol Steam Reforming over Ni/Al2O3: Influence of Nickel Precursors. ACS Sustain. Chem. Eng. 2013, 1, 1052–1062. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Marinoiu, A.; Ionita, G.; Gaspar, C.L.; Cobzaru, C.; Oprea, S. Glycerol hydrogenolysis to propylene glycol. React. Kinet. Catal. Lett. 2009, 97, 315–320. [Google Scholar] [CrossRef]
- Menchavez, R.N.; Morra, M.J.; He, B.B. Co-Production of Ethanol and 1,2-Propanediol via Glycerol Hydrogenolysis Using Ni/Ce–Mg Catalysts: Effects of Catalyst Preparation and Reaction Conditions. Catalysts 2017, 7, 290. [Google Scholar] [CrossRef] [Green Version]
- Long, W.; Hao, F.; Xiong, W.; Liu, P.; Luo, H. Modified sepiolite supported nickel and tungsten oxide catalysts for glycerol hydrogenolysis to 1,2-propanediol with high selectivity under mild conditions. React. Kinet. Mech. Catal. 2017, 122, 85–100. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P. Selective Hydrogenolysis of Glycerol with Raney Nickel. Ind. Eng. Chem. Res. 2005, 44, 8535–8537. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yu, W.; Chen, C.; Miao, H.; Ma, H.; Xu, J. Ni/NaX: A Bifunctional Efficient Catalyst for Selective Hydrogenolysis of Glycerol. Catal. Lett. 2010, 134, 184–189. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Zhou, Y.; Fu, H.; Chen, H.; Li, X. Effect of Base Additives on the Selective Hydrogenolysis of Glycerol over Ru/TiO2 Catalyst. Chem. Lett. 2007, 36, 1274–1275. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Fernandez, S.G.; Requies, J.; Doukkali, M.E.; Guemez, M.B. Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol. Catal. Today 2012, 195, 22–31. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Sawhill, S.; Layman, K.; Vanwyk, D.; Engelhard, M.; Wang, C.; Bussell, M. Thiophene hydrodesulfurization over nickel phosphide catalysts: Effect of the precursor composition and support. J. Catal. 2005, 231, 300–313. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Li, L.; Li, K. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Chu, Y.; Chen, J. Hydroconversion of Methyl Laurate as a Model Compound to Hydrocarbons on Bifunctional Ni2P/SAPO-11: Simultaneous Comparison with the Performance of Ni/SAPO-11. Energy Fuels 2014, 28, 7122–7132. [Google Scholar] [CrossRef]
- Scanlon, J.T.; Willis, D.E. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985, 23, 333–340. [Google Scholar] [CrossRef]




| Support | BET | Potentiometric Titration | IPA Decomposition Reaction (XIPA = 15 %) | |||||
|---|---|---|---|---|---|---|---|---|
| SBET a | Vp b | Ei c | NS d | T e | Spropylene f | Sacetone g | SDIPE h | |
| γ-Al2O3 | 185 | 0.50 | 60 | 0.35 | 200 | 73 | 0 | 27 |
| CS-P | 235 | 0.70 | 340 | 0.59 | 170 | 100 | 0 | 0 |
| Catalyst | AAS | BET | Potentiometric Titration | XRD | TEM | TPR | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni a | SBET b | Vp c | Ei d | NS e | dXRD f | dva g | T1 h | T2 h | T3 h | |
| Ni/γ-Al2O3 | 4.8 | 174 | 0.50 | 36 | 0.30 | n.d. | 4 | 322 | 532 | 625 |
| Ni/CS-P | 5.2 | 395 | 0.80 | 118 | 0.50 | 11 | 12 | - | - | - |
| Parameter | Value |
|---|---|
| pH | 6.0 |
| Density | 1.257 |
| Glycerol content (wt.%) | 79.3 |
| Methanol content (wt.%) | 2.0 |
| MONG (wt.%) | 6.7 |
| Water content (wt.%) | 11.0 |
| Ash content (wt.%) | 3.0 |
| Test | Gly (wt.%) | T(°C) | t(h) | X (%) | Selectivity (%) | CB c (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gases | MeOH | EtOH | AcO | 1-POH | AcCH2OH | EG | 1,2-PG | ||||||
| Ni/γ-Al2O3 catalyst | |||||||||||||
| 1 | 10 | 200 | 2 | 10.0 | 0.0 | 1.2 | 3.6 | 0.0 | 0.4 | 0.5 | 19.2 | 75.1 | 93 |
| 2 | 10 | 220 | 2 | 22.0 | 1.8 | 0.9 | 6.8 | 0.0 | 0.0 | 0.3 | 12.6 | 77.6 | 95 |
| 3 | 10 | 260 | 2 | 44.7 | 20.1 | 10.7 | 20.4 | 5.7 | 6.5 | 0.2 | 26.8 | 9.6 | 96 |
| 4 | 80 | 220 | 2 | 40.0 | 1.3 | 0.4 | 5.2 | 0.0 | 0.3 | 2.1 | 4.0 | 86.7 | 96 |
| 5 | 80 | 220 | 5 | 100.0 | 3.0 | 0.7 | 4.0 | 1.0 | 1.2 | 0.2 | 6.8 | 83.0 | 97 |
| 6 | 80 a | 220 | 5 | 100.0 | 3.2 | 0.5 | 2.4 | 0.1 | 0.1 | 0.0 | 6.7 | 87.0 | 97 |
| Ni/CS-P catalyst | |||||||||||||
| 7 | 10 | 220 | 2 | 4.9 | 0.5 | 0.1 | 1.0 | 0.3 | 19.3 | 0.8 | 0.0 | 78.0 | 96 |
| 8 | 30 | 260 | 2 | 37.9 | 1.5 | 0.3 | 4.4 | 19.8 | 64.5 | 5.3 | 0.0 | 4.2 | 96 |
| 9 | 80 | 260 | 2 | 45.1 | 1.3 | 0.4 | 4.0 | 22.0 | 67.0 | 4.0 | 0.0 | 1.3 | 96 |
| 10 | 80 a | 260 | 5 | 100.0 | 1.1 | 0.1 | 3.7 | 23.0 | 71.0 | 1.0 | 0.0 | 0.0 | 97 |
| 11 | 80 b | 260 | 2 | 100.0 | 1.5 | 0.0 | 1.1 | 2.3 | 91.0 | 4.4 | 0.0 | 0.0 | 97 |
| Ni/γ-Al2O3 catalyst + Ni/CS-P catalyst Two consecutive reaction tests: 5 h 220 °C with Ni/γ-Al2O3 catalyst followed by 2 h at 260 °C with Ni/CS-P catalyst | |||||||||||||
| 12 | 80 a | 220–260 | 7 | 100.0 | 6.0 | 1.4 | 4.5 | 2.4 | 79.3 | 0.0 | 6.5 | 0.0 | 97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.N.; Cerioni, J.L.; Pompeo, F.; Santori, G.F.; Nichio, N.N. High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts. Catalysts 2020, 10, 615. https://doi.org/10.3390/catal10060615
Gatti MN, Cerioni JL, Pompeo F, Santori GF, Nichio NN. High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts. Catalysts. 2020; 10(6):615. https://doi.org/10.3390/catal10060615
Chicago/Turabian StyleGatti, Martín N., Julieta L. Cerioni, Francisco Pompeo, Gerardo F. Santori, and Nora N. Nichio. 2020. "High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts" Catalysts 10, no. 6: 615. https://doi.org/10.3390/catal10060615