Preparation and Characterization of Photoactive Anatase TiO2 from Algae Bloomed Surface Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coagulation Efficiency of TiCl4 Comparing PAC
2.2. Physicochemical Properties of the Prepared A-TiO2
2.3. Photocatalytic Activity of Prepared A-TiO2 NPs
- Firstly, the apparent density of A-TiO2 was estimated as 1.2 g/mL, which is approximately six times larger than that of P25 (0.19 g/mL), causing a small number of adsorption sites for A-TiO2. However, when considering the substantial difference in the available adsorption sites, the reduction in maximum removal of acetaldehyde and NOx was only 11% and 20% respectively, when comparing A-TiO2 to P25 NPs.
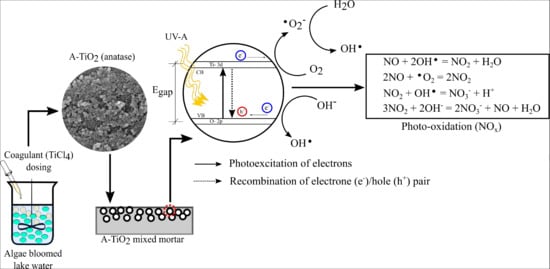
- Secondly, it is evident that doped metals and nonmetal significantly affect the photo activity of a photocatalyst [23]. The EDX analysis of the current study showed notable amounts of Ca (3.3%) and Cl (2.7%) in A-TiO2. Castro and Durán [53] reported that Ca doping on TiO2 at a very low concentration (<3 wt.%) could reduce the band energy gap of TiO2 and enhance the photodegradation of methyl orange under solar irradiation. However, the doped Ca of substantial amount can act as recombination site for e-/h+ pair generated during photodegradation (see Figure 1) and might cause a reduced level of photoactivity [32]. Similarly, Wang et al. [54] illustrated that a certain amount (2 atomic%–4 atomic%) of Cl as the dopant in TiO2 could activate TiO2 under visible light, and the extent of light absorption within the UV range can get reduced, so as the photoactivity. Hence, in the current study, the dual effect of the doped Ca and Cl might have reduced the photocatalytic activity of A-TiO2.
2.4. Photocatalytic Activity of Prepared A-TiO2 NPs
3. Materials and Methods
3.1. Chemical Reagents and Simulated Algal Wastewater
3.2. Jar Tests
3.3. Preparation and Characterization of TiO2 from Flocculated Algal Sludge
3.4. Preparation of A-TiO2 Mixed Mortar Blocks
3.5. Evaluation of Photocatalytic Activities of Prepared A-TiO2
3.5.1. Removal of Acetaldehyde under UV Irradiation
3.5.2. Removal of NOx under UV Irradiation
4. Conclusions
- When the coagulant dose varied from 0.1 to 0.3 g/L in algae enriched wastewater, TiCl4 was found to be superior in removing turbidity, COD, and TP when compared with commercially available PAC. More importantly, TiCl4 removed almost 97% of the effluent TP at a coagulant dose of 0.3 g/L.
- The prepared A-TiO2 NPs effectively removed 85.7% of gaseous acetaldehyde under UV-A exposure for 120 min., and by considering the pseudo 1st order kinetic reaction, the reaction rate constant was found as 0.0169 min−1, which is approximately 54.34% of commercially available P25. Additionally, in a continuous flow reaction, under UV-A irradiation for 60 min., the as-prepared A-TiO2 NPs was found to remove approximately 28% of NO, on average. The A-TiO2 mixed mortar blocks prepared in this study showed 50% less NOx removal efficiency when compared to P25 mixed mortar blocks under UV irradiance.
Author Contributions
Funding
Conflicts of Interest
References
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Naceradska, J.; Novotna, K.; Cermakova, L.; Cajthaml, T.; Pivokonsky, M. Investigating the coagulation of non-proteinaceous algal organic matter: Optimizing coagulation performance and identification of removal mechanisms. J. Environ. Sci. China 2019, 79, 25–34. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Lee, S.; Kim, H.-G.; Zhao, D.; Park, J.-A.; Choi, J.-W. Organic/inorganic hybrid adsorbent for efficient phosphate removal from a reservoir affected by algae bloom. J. Ind. Eng. Chem. 2019, 69, 211–216. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Kim, C.G. Comparison of algal removal by coagulation with clays and Al-based coagulants. Sep. Sci. Technol. 2008, 43, 1677–1686. [Google Scholar] [CrossRef]
- Chen, J.J.; Yeh, H.H.; Tseng, I.C. Effect of ozone and permanganate on algae coagulation removal-pilot and bench scale tests. Chemosphere 2009, 74, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yu, C.; Zhao, Y.; Fan, M.; Gao, B. The removal of silver nanoparticle by titanium tetrachloride and modified sodium alginate composite coagulants: Floc properties, membrane fouling, and floc recycle. Environ. Sci. Pollut. Res. 2018, 25, 21058–21069. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae. Water Res. 2010, 44, 3617–3624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghernaout, B.; Ghernaout, D.; Saiba, A. Algae and cyanotoxins removal by coagulation/flocculation: A review. Desalination Water Treat. 2012, 20, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Xu, Y.; Sun, W.; Sun, Y.; Zheng, H. UV-initiated synthesis of a novel chitosan-based flocculant with high flocculation efficiency for algal removal. Sci. Total Environ. 2017, 609, 410–418. [Google Scholar] [CrossRef]
- Ma, J.; Liu, W. Effectiveness and mechanism of potassium ferrate(VI) preoxidation for algae removal by coagulation. Water Res. 2002, 36, 871–878. [Google Scholar] [CrossRef]
- Shen, Q.; Zhu, J.; Cheng, L.; Zhang, J.; Zhang, Z.; Xu, X. Enhanced algae removal by drinking water treatment of chlorination coupled with coagulation. Desalination 2011, 271, 236–240. [Google Scholar] [CrossRef]
- Liu, L.; Chu, X.; Chen, P.; Xiao, Y.; Hu, J. Effects of water quality on inactivation and repair of Microcystis viridis and Tetraselmis suecica following medium-pressure UV irradiation. Chemosphere 2016, 163, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Cao, Z.; Chen, W.; Bi, H. Variation of dissolved organic nitrogen concentration during the ultrasonic pretreatment to Microcystis aeruginosa. Ultrason. Sonochem. 2016, 29, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Gao, B.; Zhao, Q. Enhanced algae removal by Ti-based coagulant: Comparison with conventional Al- and Fe-based coagulants. Environ. Sci. Pollut. Res. 2018, 25, 13147–13158. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Kim, G.J.; Kim, J.B.; Kim, J.H. Preparation of titanium dioxide (TiO2) from sludge produced by titanium tetrachloride (TiCl4) flocculation of wastewater. Environ. Sci. Technol. 2007, 41, 1372–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.X.; Gao, B.Y.; Zhang, G.Z.; Qi, Q.B.; Wang, Y.; Phuntsho, S.; Kim, J.H.; Shon, H.K.; Yue, Q.Y.; Li, Q.; et al. Coagulation and sludge recovery using titanium tetrachloride as coagulant for real water treatment: A comparison against traditional aluminum and iron salts. Sep. Purif. Technol. 2014, 130, 19–27. [Google Scholar] [CrossRef]
- Chekli, L.; Eripret, C.; Park, S.H.; Tabatabai, S.A.A.; Vronska, O.; Tamburic, B.; Kim, J.H.; Shon, H.K. Coagulation performance and floc characteristics of polytitanium tetrachloride (PTC) compared with titanium tetrachloride (TiCl4) and ferric chloride (FeCl3) in algal turbid water. Sep. Purif. Technol. 2017, 175, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Chekli, L.; Corjon, E.; Tabatabai, S.A.A.; Naidu, G.; Tamburic, B.; Park, S.H.; Shon, H.K. Performance of titanium salts compared to conventional FeCl3 for the removal of algal organic matter (AOM) in synthetic seawater: Coagulation performance, organic fraction removal and floc characteristics. J. Environ. Manag. 2017, 201, 28–36. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Li, X.-Q.; Ma, C.-X.; Pei, R.-T. The cell damage of Microcystis aeruginosa in PACl coagulation and floc storage processes. Sep. Purif. Technol. 2013, 115, 123–128. [Google Scholar] [CrossRef]
- El Saliby, I.J.; Shon, H.K.; Okour, Y.H.; Vigneswaran, S.; Senthilnanthanan, M.; Kandasamy, J. Production of titanium dioxide nanoparticles and nanostructures from dye wastewater sludge-characterisation and evaluation of photocatalytic activity. J. Adv. Oxid. Technol. 2010, 13, 15–20. [Google Scholar] [CrossRef]
- Na, S.-H.; Shon, H.K.; Kim, J.B.; Park, H.J.; Kim, J.-H. Preparation and characterization of titania nanoparticle produced from Ti-flocculated sludge with paper mill wastewater. J. Ind. Eng. Chem. 2011, 17, 277–281. [Google Scholar] [CrossRef]
- Shon, H.; Okour, Y.; El Saliby, I.; Park, J.; Cho, D.; Kim, J.-B.; Park, H.-J.; Kim, J.-H. Preparation and characterisation of titanium dioxide produced from Ti-salt flocculated sludge in water treatment. J. Korean Ind. Eng. Chem. 2009, 11, 1453–1458. [Google Scholar]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [Green Version]
- Gallus, M.; Ciuraru, R.; Mothes, F.; Akylas, V.; Barmpas, F.; Beeldens, A.; Bernard, F.; Boonen, E.; Boreave, A.; Cazaunau, M.; et al. Photocatalytic abatement results from a model street canyon. Environ. Sci. Pollut. Res. Int. 2015, 22, 18185–18196. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef]
- Chen, X.-F.; Lin, S.-r.; Kou, S.-C. Effect of composite photo-catalysts prepared with recycled clay brick sands and nano-TiO2 on methyl orange and NOx removal. Constr. Build. Mater. 2018, 171, 152–160. [Google Scholar] [CrossRef]
- McConnell, R.; Islam, T.; Shankardass, K.; Jerrett, M.; Lurmann, F.; Gilliland, F.; Gauderman, J.; Avol, E.; Kunzli, N.; Yao, L.; et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ. Health Perspect. 2010, 118, 1021–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.; Yun, T.S. NOx removal rate of photocatalytic cementitious materials with TiO2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Angelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Karapati, S.; Giannakopoulou, T.; Todorova, N.; Boukos, N.; Antiohos, S.; Papageorgiou, D.; Chaniotakis, E.; Dimotikali, D.; Trapalis, C. TiO2 functionalization for efficient NOx removal in photoactive cement. Appl. Surf. Sci. 2014, 319, 29–36. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Photocatalytic roads: From lab tests to real scale applications. Eur. Transp. Res. Rev. 2012, 5, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Ohama, Y.; Van Gemert, D. Applications of Titanium Dioxide Photocatalysis to Construction Materials; Springer: Dordrecht, The Netherlands, 2011; Volume 5. [Google Scholar]
- Boonen, E.; Beeldens, A. Recent photocatalytic applications for air purification in belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Hong, S.J.; Kim, H.B.; Lee, S.W. Evaluation of in-situ NOx removal efficiency of photocatalytic concrete in expressways. KSCE J. Civ. Eng. 2017, 22, 2274–2280. [Google Scholar] [CrossRef]
- Li, F.; Jiang, J.-Q.; Wu, S.; Zhang, B. Preparation and performance of a high purity poly-aluminum chloride. Chem. Eng. J. 2010, 156, 64–69. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Zhao, C.; Wang, Y.; Xie, W.; Chen, W.; Yang, C. An alternative method for preparation of polyaluminum chloride coagulant using fresh aluminum hydroxide gels: Characterization and coagulation performance. Chem. Eng. Res. Des. 2015, 104, 208–217. [Google Scholar] [CrossRef]
- Jeon, K.-J.; Ahn, J.-H. Evaluation of titanium tetrachloride and polytitanium tetrachloride to remove phosphorus from wastewater. Sep. Purif. Technol. 2018, 197, 197–201. [Google Scholar] [CrossRef]
- Zhang, W.; Song, R.; Cao, B.; Yang, X.; Wang, D.; Fu, X.; Song, Y. Variations of floc morphology and extracellular organic matters (EOM) in relation to floc filterability under algae flocculation harvesting using polymeric titanium coagulants (PTCs). Bioresour. Technol. 2018, 256, 350–357. [Google Scholar] [CrossRef]
- Jeon, K.J.; Kim, J.H.; Ahn, J.H. Phosphorus removal characteristics of titanium salts compared with aluminum salt. Water Environ. Res. 2017, 89, 739–743. [Google Scholar] [CrossRef]
- Park, S.M.; Chekli, L.; Kim, J.B.; Shahid, M.; Shon, H.K.; Kim, P.S.; Lee, W.-S.; Lee, W.E.; Kim, J.-H. NO removal of mortar mixed with titania produced from Ti-salt flocculated sludge. J. Ind. Eng. Chem. 2014, 20, 3851–3856. [Google Scholar] [CrossRef]
- El Saliby, I.; Okour, Y.; Shon, H.K.; Kandasamy, J.; Lee, W.E.; Kim, J.-H. TiO2 nanoparticles and nanofibres from TiCl4 flocculated sludge: Characterisation and photocatalytic activity. J. Ind. Eng. Chem. 2012, 18, 1033–1038. [Google Scholar] [CrossRef]
- Shon, H.K.; Okour, Y.; Park, S.M.; Kim, J.B.; Kim, J.H. Titania produced from Ti-salt flocculated sludge: Photocatalytic activity under solar light. J. Nanosci. Nanotechnol. 2014, 14, 6386–6389. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.M.; Liu, G.J. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 2019, 215, 101–114. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Okour, Y.; El Saliby, I.; Shon, H.K.; Vigneswaran, S.; Kim, J.H.; Cho, J.; Kim, I.S. Recovery of sludge produced from Ti-salt flocculation as pretreatment to seawater reverse osmosis. Desalination 2009, 247, 53–63. [Google Scholar] [CrossRef]
- Chi, Y.; Tian, C.; Li, H.; Zhao, Y. Polymerized titanium salts for algae-laden surface water treatment and the algae-rich sludge recycle toward chromium and phenol degradation from aqueous solution. ACS Sustain. Chem. Eng. 2019, 7, 12964–12972. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Kamińska, A.; Łomot, D. Dual functionality of TiO2/biochar hybrid materials: Photocatalytic phenol degradation in the liquid phase and selective oxidation of methanol in the gas phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef] [Green Version]
- Karafas, E.S.; Romanias, M.N.; Stefanopoulos, V.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Papagiannakopoulos, P. Effect of metal doped and co-doped TiO2 photocatalysts oriented to degrade indoor/outdoor pollutants for air quality improvement. A kinetic and product study using acetaldehyde as probe molecule. J. Photochem. Photobiol. A-Chem. 2019, 371, 255–263. [Google Scholar] [CrossRef]
- Castro, Y.; Durán, A. Ca doping of mesoporous TiO2 films for enhanced photocatalytic efficiency under solar irradiation. J. Sol-Gel Sci. Technol. 2016, 78, 482–491. [Google Scholar] [CrossRef]
- Wang, X.-K.; Wang, C.; Jiang, W.-Q.; Guo, W.-L.; Wang, J.-G. Sonochemical synthesis and characterization of Cl-doped TiO2 and its application in the photodegradation of phthalate ester under visible light irradiation. Chem. Eng. J. 2012, 189–190, 288–294. [Google Scholar] [CrossRef]
- El Zein, A.; Bedjanian, Y.; Romanias, M.N. Kinetics and products of HONO interaction with TiO2 surface under UV irradiation. Atmos. Environ. 2013, 67, 203–210. [Google Scholar] [CrossRef]
- ISO. ISO 679: Cement Test Methods–Determination of Strength; ISO: Geneva, Switzerland, 2009. [Google Scholar]









| Dosage (g/L) | Ti | Al | ||||
|---|---|---|---|---|---|---|
| Turbidity (NTU) | COD (mg/L) | TP (mg/L) | Turbidity (NTU) | COD (mg/L) | TP (mg/L) | |
| 0.0 | 500 ± 0.018 | 117 ± 0.503 | 3.61 ± 0.261 | 500 ± 0.018 | 117 ± 0.503 | 3.61 ± 0.261 |
| 0.1 | 75 ± 0.050 | 115 ± 0.362 | 1.08 ± 0.251 | 290 ± 0.052 | 113 ± 0.233 | 1.81 ± 0.050 |
| 0.2 | 1.9 ± 0.178 | 115 ± 0.308 | 0.59 ± 0.122 | 65 ± 0.051 | 84 ± 0.328 | 0.32 ± 0.102 |
| 0.3 | 1.0 ± 0.156 | 39 ± 0.135 | 0.11 ± 0.051 | 21 ± 0.044 | 86 ± 0.244 | 0.16 ± 0.057 |
| Material | Weight (%) | SBET (m2/g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Fe | Ti | ||
| A-Residue | 45.5 | 2.0 | 3.8 | 4.4 | 13.7 | 3.5 | 3.1 | 2.4 | 5.0 | 11.1 | 5.5 | - | 9 |
| A-TiO2 | 49.3 | 1.9 | 1.3 | - | 0.6 | - | 1.1 | 2.7 | 0.6 | 3.3 | - | 39.2 | 40 |
| Sample | NO(initial) | NO(removal) | %NO(removal) | NO2(initial) | NO2(production) | %NO2(production) |
|---|---|---|---|---|---|---|
| - | µmol | µmol | - | µmol | µmol | - |
| NPs | ||||||
| A-TiO2 | 6.95 | 1.95 | 27.99% | 0.45 | 1.15 | 16.50% |
| P25 | 7.01 | 3.80 | 54.17% | 0.65 | 1.39 | 19.79% |
| Mortar blocks | ||||||
| A-TiO2 (5%) | 7.46 | 0.24 | 3.24% | 0.03 | 0.08 | 1.08% |
| A-TiO2 (10%) | 7.49 | 0.46 | 6.14% | 0.01 | 0.13 | 1.78% |
| P25 (5%) | 7.45 | 0.45 | 6.02% | 0.02 | 0.14 | 1.88% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, S.M.; Park, H.; Kang, H.-J.; Kim, J.B.; Tijing, L.; Rhee, I.; Jun, Y.-S.; Shon, H.K.; Kim, J.-H. Preparation and Characterization of Photoactive Anatase TiO2 from Algae Bloomed Surface Water. Catalysts 2020, 10, 452. https://doi.org/10.3390/catal10040452
Hossain SM, Park H, Kang H-J, Kim JB, Tijing L, Rhee I, Jun Y-S, Shon HK, Kim J-H. Preparation and Characterization of Photoactive Anatase TiO2 from Algae Bloomed Surface Water. Catalysts. 2020; 10(4):452. https://doi.org/10.3390/catal10040452
Chicago/Turabian StyleHossain, Sayed Mukit, Heeju Park, Hui-Ju Kang, Jong Beom Kim, Leonard Tijing, Inkyu Rhee, Young-Si Jun, Ho Kyong Shon, and Jong-Ho Kim. 2020. "Preparation and Characterization of Photoactive Anatase TiO2 from Algae Bloomed Surface Water" Catalysts 10, no. 4: 452. https://doi.org/10.3390/catal10040452