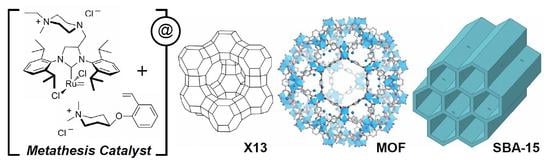
Preparation of Ruthenium Olefin Metathesis Catalysts Immobilized on MOF, SBA-15, and 13X for Probing Heterogeneous Boomerang Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tagged Ligand Precursor and Catalyst Synthesis
2.2. Supports and Catalysts Immobilization
2.3. Optimization of Metathesis Reaction Conditions
2.4. Reuse of Heterogeneous Catalysts
2.5. Substrate Scope and Limitations Study
2.6. Probing the Boomerang Existence
3. Materials and Methods
3.1. General
3.2. Synthesis of Complexes 11 and 6
3.3. Catalysts and Ligand Immobilization
3.4. Catalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grela, K. Olefin Metathesis: Theory and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Kingsbury, J.S.; Hoveyda, A.H. Regarding the Mechanism of Olefin Metathesis with Sol−Gel-Supported Ru-Based Complexes Bearing a Bidentate Carbene Ligand. Spectroscopic Evidence for Return of the Propagating Ru Carbene. J. Am. Chem. Soc. 2005, 127, 4510–4517. [Google Scholar] [CrossRef] [PubMed]
- Michrowska, A.; Bujok, R.; Harutyunyan, S.; Sashuk, V.; Dolgonos, G.; Grela, K. Nitro-Substituted Hoveyda−Grubbs Ruthenium Carbenes: Enhancement of Catalyst Activity through Electronic Activation. J. Am. Chem. Soc. 2004, 126, 9318–9325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieniek, M.; Bujok, R.; Stępowska, H.; Jacobi, A.; Hagenkötter, R.; Arlt, D.; Jarzembska, K.; Makal, A.; Woźniak, K.; Grela, K. New air-stable ruthenium olefin metathesis precatalysts derived from bisphenol S. J. Organomet. Chem. 2006, 691, 5289–5297. [Google Scholar] [CrossRef]
- Rix, D.; Caijo, F.; Laurent, I.; Boeda, F.; Clavier, H.; Nolan, S.P.; Mauduit, M. Aminocarbonyl Group Containing Hoveyda−Grubbs-Type Complexes: Synthesis and Activity in Olefin Metathesis Transformations. J. Org. Chem. 2008, 73, 4225–4228. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, M.; Michrowska, A.; Usanov, D.L.; Grela, K. In an Attempt to Provide a User’s Guide to the Galaxy of Benzylidene, Alkoxybenzylidene, and Indenylidene Ruthenium Olefin Metathesis Catalysts. Chem.—Eur. J. 2008, 14, 806–818. [Google Scholar] [CrossRef]
- Vorfalt, T.; Wannowius, K.J.; Thiel, V.; Plenio, H. How Important Is the Release–Return Mechanism in Olefin Metathesis? Chem. Eur. J. 2010, 16, 12312–12315. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Bieniek, M.; Skowerski, K.; Grela, K. A New Tool in the Toolbox: Electron-Withdrawing Group Activated Ruthenium Catalysts for Olefin Metathesis. Synlett 2013, 24, 903–919. [Google Scholar] [CrossRef]
- Bates, J.M.; Lummiss, J.A.M.; Bailey, G.A.; Fogg, D.E. Operation of the Boomerang Mechanism in Olefin Metathesis Reactions Promoted by the Second-Generation Hoveyda Catalyst. ACS Catal. 2014, 4, 2387–2394. [Google Scholar] [CrossRef]
- Michrowska, A.; Mennecke, K.; Kunz, U.; Kirschning, A.; Grela, K. A New Concept for the Noncovalent Binding of a Ruthenium-Based Olefin Metathesis Catalyst to Polymeric Phases: Preparation of a Catalyst on Raschig Rings. J. Am. Chem. Soc. 2006, 128, 13261–13267. [Google Scholar] [CrossRef]
- Buchmeiser, M.R. Polymer-Supported Well-Defined Metathesis Catalysts. Chem. Rev. 2009, 109, 303–321. [Google Scholar] [CrossRef]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-Based Olefin Metathesis Catalysts Bearing N-Heterocyclic Carbene Ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef] [PubMed]
- Skowerski, K.; Białecki, J.; Czarnocki, S.J.; Żukowska, K.; Grela, K. Effective immobilization of a metathesis catalyst bearing an ammonium-tagged NHC ligand on various solid supports. Beilstein J. Org. Chem. 2016, 12, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Skowerski, K.; Pastva, J.; Czarnocki, S.J.; Janoscova, J. Exceptionally Stable and Efficient Solid Supported Hoveyda-Type Catalyst. Org. Process Res. Dev. 2015, 19, 872–877. [Google Scholar] [CrossRef]
- Jana, A.; Grela, K. Forged and fashioned for faithfulness—Ruthenium olefin metathesis catalysts bearing ammonium tags. Chem. Commun. 2018, 54, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.apeiron-synthesis.com/katalizatory/fixcat/ (accessed on 15 March 2020).
- Skowerski, K.; Szczepaniak, G.; Wierzbicka, C.; Gułajski, Ł.; Bieniek, M.; Grela, K. Highly active catalysts for olefin metathesis in water. Catal. Sci. Technol. 2012, 2, 2424–2427. [Google Scholar] [CrossRef]
- Bujok, R.; Bieniek, M.; Masnyk, M.; Michrowska, A.; Sarosiek, A.; Stȩpowska, H.; Arlt, D.; Grela, K. Ortho- and Para-Substituted Hoveyda−Grubbs Carbenes. An Improved Synthesis of Highly Efficient Metathesis Initiators. J. Org. Chem. 2004, 69, 6894–6896. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sigmaaldrich.com/chemistry/chemical-synthesis/learning-center/technical-bulletins/al-1430/molecular-sieves.html (accessed on 15 March 2020).
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Girgsdies, F.; Schlögl, R.; Hess, C. Pore structure and surface area of silica SBA-15: Influence of washing and scale-up. Beilstein J. Nanotechnol. 2011, 2, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Chołuj, A.; Zieliński, A.; Grela, K.; Chmielewski, M.J. Metathesis@MOF: Simple and Robust Immobilization of Olefin Metathesis Catalysts inside (Al)MIL-101-NH2. ACS Catal. 2016, 6, 6343–6349. [Google Scholar] [CrossRef]
- Bek, D.; Balcar, H.; Žilková, N.; Zukal, A.; Horáček, M.; Čejka, J. Grubbs Catalysts Immobilized on Mesoporous Molecular Sieves via Phosphine and Pyridine Linkers. ACS Catal. 2011, 1, 709–718. [Google Scholar] [CrossRef]
- Ritter, T.; Hejl, A.; Wenzel, A.G.; Funk, T.W.; Grubbs, R.H. A Standard System of Characterization for Olefin Metathesis Catalysts. Organometallics 2006, 25, 5740–5745. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, M.; Wyrębek, P.; Trzybiński, D.; Woźniak, K.; Grela, K. In a Quest for Selectivity Paired with Activity: A Ruthenium Olefin Metathesis Catalyst Bearing an Unsymmetrical Phenanthrene-Based N-Heterocyclic Carbene. Chem.—Eur. J. 2020, 26, 3782–3794. [Google Scholar]
- Małecki, P.; Gajda, K.; Gajda, R.; Woźniak, K.; Trzaskowski, B.; Kajetanowicz, A.; Grela, K. Specialized Ruthenium Olefin Metathesis Catalysts Bearing Bulky Unsymmetrical NHC Ligands: Computations, Synthesis, and Application. ACS Catal. 2019, 9, 587–598. [Google Scholar] [CrossRef]
- Smoleń, M.; Kośnik, W.; Gajda, R.; Woźniak, K.; Skoczeń, A.; Kajetanowicz, A.; Grela, K. Ruthenium Complexes Bearing Thiophene-Based Unsymmetrical N-Heterocyclic Carbene Ligands as Selective Catalysts for Olefin Metathesis in Toluene and Environmentally Friendly 2-Methyltetrahydrofuran. Chem.—Eur. J. 2018, 24, 15372–15379. [Google Scholar]
- Rouen, M.; Queval, P.; Borré, E.; Falivene, L.; Poater, A.; Berthod, M.; Hugues, F.; Cavallo, L.; Baslé, O.; Olivier-Bourbigou, H.; et al. Selective Metathesis of α-Olefins from Bio-Sourced Fischer–Tropsch Feeds. ACS Catal. 2016, 6, 7970–7976. [Google Scholar] [CrossRef]
- Czaban, J.; Schertzer, B.M.; Grela, K. Low Catalyst Loadings in Self-Metathesis of 1-Dodecene. Adv. Synth. Catal. 2013, 355, 1997–2006. [Google Scholar] [CrossRef]
- Chen, G.-W.; Kirschning, A. First Preparation of Spacer-Linked Cyclic Neooligoaminodeoxysaccharides. Chem.—Eur. J. 2002, 8, 2717–2729. [Google Scholar] [CrossRef]
- Scanlon, J.T.; Willis, D.E. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985, 23, 333–340. [Google Scholar] [CrossRef]
- BouzBouz, S.; Boulard, L.; Cossy, J. Ruthenium-Catalyzed Cross-Metathesis between Diallylsilanes and Electron-Deficient Olefins. Org. Lett. 2007, 9, 3765–3768. [Google Scholar] [CrossRef]
- So, C.M.; Kume, S.; Hayashi, T. Rhodium-Catalyzed Asymmetric Hydroarylation of 3-Pyrrolines Giving 3-Arylpyrrolidines: Protonation as a Key Step. J. Am. Chem. Soc. 2013, 135, 10990–10993. [Google Scholar] [CrossRef]
- Coleman, G.H.; Callen, J.E.; Dornfeld, C.A. Syntheses of δ-Cyclopentyl-n-valeric Acid1. J. Am. Chem. Soc. 1946, 68, 1101–1102. [Google Scholar] [CrossRef]
- Fu, M.; Chen, L.; Jiang, Y.; Jiang, Z.-X.; Yang, Z. Copper-Catalyzed Intermolecular Chloro- and Bromotrifluoromethylation of Alkenes. Org. Lett. 2016, 18, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, G.; Urbaniak, K.; Wierzbicka, C.; Kosiński, K.; Skowerski, K.; Grela, K. High-Performance Isocyanide Scavengers for Use in Low-Waste Purification of Olefin Metathesis Products. ChemSusChem 2015, 8, 4139–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Catalyst/Support | Catalyst Loading on Support (wt %) | |||
|---|---|---|---|---|
| 1.0 | 0.5 | 0.1 | 0.05 | |
| 4@13X | 9 400 | 10 000 | 7 500 | 4 400 |
| 4@SBA-15 | 8 000 | 12 800 | 12 800 | 12 800 |
| 4@MOF | 17 800 | 19 700 | 21 600 | 24 100 |
| Entry | Catalyst@support | 5 (equiv.) | Conversion (%) | Turnover Number (TON) |
|---|---|---|---|---|
| 1 | 4@13X | 0 | 50 | 10,000 |
| 1 | 15 | 3000 | ||
| 10 | 27 | 5400 | ||
| 20 | 21 | 4200 | ||
| 2 | 6@13X | 0 | 41 | 8200 |
| 1 | 42 | 8400 | ||
| 10 | 30 | 6000 | ||
| 20 | 25 | 5000 | ||
| 3 | 4@SBA-15 | 0 | 64 | 12,800 |
| 1 | 40 | 8000 | ||
| 10 | 30 | 6000 | ||
| 20 | 15 | 3000 | ||
| 4 | 6@SBA-15 | 0 | 70 | 14,000 |
| 1 | 68 | 13,600 | ||
| 10 | 49 | 9800 | ||
| 20 | 31 | 6200 | ||
| 5 | 4@MOF | 0 | 91 | 18,200 |
| 1 | 90 | 18,000 | ||
| 10 | 83 | 16,600 | ||
| 20 | 81 | 16,200 | ||
| 60 | 57 | 11,400 | ||
| 6 | 6@MOF | 0 | 33 | 6600 |
| 20 | 13 | 2600 | ||
| 100 | 4 | 800 |
| Entry | Substrate | Catalyst (Loading) | Conv. (Selec.)2 (%) | E/Z Ratio | TON | TOF (min−1)3 |
|---|---|---|---|---|---|---|
| 1 |  | 4@MOF (50 ppm) | 95 (>99) | - | 19 000 | 370 |
| 6@MOF (50 ppm) | 47 (>99) | - | 9 400 | 93 | ||
| 4@SBA-15 (50 ppm) | 83 (>99) | - | 16 600 | 209 | ||
| 6@SBA-15 (50 ppm) | 79 (>99) | - | 15 800 | 99 | ||
| 2 |  | 4@MOF (50 ppm) | 86 (>99) | - | 17 200 | 320 |
| 6@MOF (50 ppm) | 58 (>99) | - | 11 600 | 160 | ||
| 4@SBA-15 (20 ppm) | 54 (>99) | - | 27 000 | 1498 | ||
| 6@SBA-15 (20 ppm) | 51 (>99) | - | 25 500 | 1528 | ||
| 3 |  | 4@SBA (50 ppm) | 29 (99) | 3.3 | 5 800 | 116 |
| 6@SBA (50 ppm) | 27 (99) | 3.2 | 5 600 | 77 | ||
| 4@MOF (100 ppm) | 65 (99) | 4.6 | 6 500 | 203 | ||
| 6@MOF (100 ppm) | 60 (99) | 4.1 | 6 000 | 93 | ||
| 4@SBA (100 ppm) | 48 (98) | 4.5 | 4 800 | nd | ||
| 6@SBA (100 ppm) | 44 (98) | 4.3 | 4 400 | nd | ||
| 4 |  | 4@MOF (50 ppm) | 57 (>99) | 3.8 | 11 400 | 207 |
| 6@MOF (50 ppm) | 46 (>99) | 3.2 | 9 200 | 33 | ||
| 4@SBA (50 ppm) | 50 (>99) | 3.2 | 10 000 | 242 | ||
| 6@SBA (50 ppm) | 51 (>99) | 3.2 | 10 500 | 211 | ||
| 4@SBA (100 ppm) | 100 (>99) | 3.9 | 10 000 | nd | ||
| 6@SBA (100 ppm) | 100 (>99) | 4.0 | 10 000 | nd | ||
| 5 |  | 4@MOF (250 ppm) | 70 (96) | 11.8 | 2 800 | 87 |
| 6@MOF (250 ppm) | 68 (95) | 10.1 | 2 700 | 40 | ||
| 4@SBA (250 ppm) | 38 (4) | 6.1 | 750 | 5 | ||
| 6@SBA (250 ppm) | 34 (3) | 5.3 | 670 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chołuj, A.; Nogaś, W.; Patrzałek, M.; Krzesiński, P.; Chmielewski, M.J.; Kajetanowicz, A.; Grela, K. Preparation of Ruthenium Olefin Metathesis Catalysts Immobilized on MOF, SBA-15, and 13X for Probing Heterogeneous Boomerang Effect. Catalysts 2020, 10, 438. https://doi.org/10.3390/catal10040438
Chołuj A, Nogaś W, Patrzałek M, Krzesiński P, Chmielewski MJ, Kajetanowicz A, Grela K. Preparation of Ruthenium Olefin Metathesis Catalysts Immobilized on MOF, SBA-15, and 13X for Probing Heterogeneous Boomerang Effect. Catalysts. 2020; 10(4):438. https://doi.org/10.3390/catal10040438
Chicago/Turabian StyleChołuj, Artur, Wojciech Nogaś, Michał Patrzałek, Paweł Krzesiński, Michał J. Chmielewski, Anna Kajetanowicz, and Karol Grela. 2020. "Preparation of Ruthenium Olefin Metathesis Catalysts Immobilized on MOF, SBA-15, and 13X for Probing Heterogeneous Boomerang Effect" Catalysts 10, no. 4: 438. https://doi.org/10.3390/catal10040438