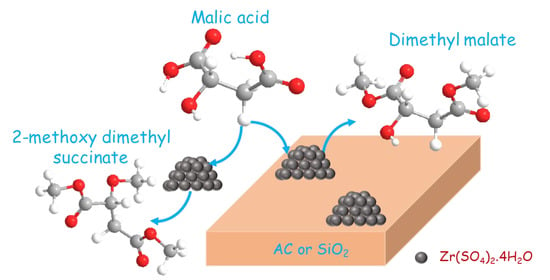
Active, Selective, and Recyclable Zr(SO4)2/SiO2 and Zr(SO4)2/Activated Carbon Solid Acid Catalysts for Esterification of Malic Acid to Dimethyl Malate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalytic Reaction
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiyokawa, S.; Kikuchi, R.; Ohashi, Y. Cosmetics e.g., Skin Cosmetics, Hair Cosmetics, and Makeup Cosmetics, Comprise Malic Acid Ester. JP2020002056-A, 9 January 2020. [Google Scholar]
- Liu, Z.; Zhu, H.; Miao, M. Synthesis Method of Malic Acid Di-Menthyl Ester Coolant Agent and Its Application in Tobacco. CN1301958-C, 28 February 2007. [Google Scholar]
- Ikejiri, Y. Malic Acid Dialkyl Ester Used as Plasticizer, Surfactant or Its Intermediary Raw Material Comprises Predetermined Oligomer Content. JP5245298-B2, 24 July 2013. [Google Scholar]
- Go, A.W.; Phuong Lan Tran, N.; Lien Huong, H.; Liu, Y.T.; Sutanto, S.; Ju, Y.H. Catalyst free esterification of fatty acids with methanol under subcritical condition. Energy 2014, 70, 393–400. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Calandruccio, C.; Salerno, R.; Procopio, A. Tunable microwave-assisted method for the solvent-free and catalyst-free peracetylation of natural products. Beilstein Org. Chem. 2016, 12, 2222–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, M.; Shestakova, P.; Lazarova, H.; Dimitrov, M.; Kovacheva, D.; Szegedi, A.; Mali, G.; Dasireddy, V.; Likozar, B.; Wilde, N.; et al. Efficient solid acid catalysts based on sulfated tin oxides for liquid phase esterification of levulinic acid with ethanol. Appl. Catal. Gen. 2018, 560, 119–131. [Google Scholar] [CrossRef]
- Ruan, H.; Xu, L.; Hong, S.; Liu, S. Highly effective solvent free esterification of phytosterols employing edible metal oxide-emulsifier as catalyst. Chem. Phys. Lipids 2018, 213, 118–123. [Google Scholar] [CrossRef]
- Ketzer, F.; Celante, D.; de Castilhos, F. Catalytic performance and ultrasonic-assisted impregnation effects on WO3/USY zeolites in esterification of oleic acid with methyl acetate. Microporous Mesoporous Mater. 2020, 291, 109704. [Google Scholar] [CrossRef]
- Kong, P.S.; Cognet, P.; Peres, Y.; Esvan, J.; Daud, W.M.A.W.; Aroua, M.K. Development of a Novel Hydrophobic ZrO2-SiO2 Based Acid Catalyst for Catalytic Esterification of Glycerol with Oleic Acid. Ind. Eng. Chem. Res. 2018, 57, 9386–9399. [Google Scholar] [CrossRef] [Green Version]
- Furuta, S.; Matsuhashi, H.; Arata, K. Catalytic action of sulfated tin oxide for etherification and esterification in comparison with sulfated zirconia. Appl. Catal. Gen. 2004, 269, 187–191. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Miyazaki, H.; Kawamura, Y.; Nakamura, H.; Arata, K. Preparation of a solid superacid of sulfated tin oxide with acidity higher than that of sulfated zirconia and its applications to aldol condensation and benzoylation. Chem. Mater. 2001, 13, 3038–3042. [Google Scholar] [CrossRef]
- Reddy, B.M.; Sreekanth, P.M.; Lakshmanan, P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation reactions. J. Mol. Catal. Chem. 2005, 237, 93–100. [Google Scholar] [CrossRef]
- Katada, N.; Endo, J.; Notsu, K.; Yasunobu, N.; Naito, N.; Niwa, M. Superacidity and catalytic activity of sulfated zirconia. J. Phys. Chem. B 2000, 104, 10321–10328. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, M.; Dong, X.; Wang, L. Preparation of sulfonated ordered mesoporous carbon catalyst and its catalytic performance for esterification of free fatty acids in waste cooking oils. RSC Adv. 2019, 9, 15941–15948. [Google Scholar]
- Pavlovic, J.; Popova, M.; Mihalyi, R.M.; Mazaj, M.; Mali, G.; Kovac, J.; Lazarova, H.; Rajic, N. Catalytic activity of SnO2-and SO4/SnO2-containing clinoptilolite in the esterification of levulinic acid. Microporous Mesoporous Mater. 2019, 279, 10–18. [Google Scholar] [CrossRef]
- Vieira, S.S.; Graca, I.; Fernandes, A.; Lopes, J.M.F.M.; Ribeiro, M.F.; Magriotis, Z.M. Influence of calcination temperature on catalytic, acid and textural properties of SO42−/La2O3/HZSM-5 type catalysts for biodiesel production by esterification. Microporous Mesoporous Mater. 2018, 270, 189–199. [Google Scholar] [CrossRef]
- Kotsarenko, N.S.; Shmachkova, V.P. Catalytic properties of the thermal decomposition products of Zr(SO4)2∙4H2O. Kinet. Catal. 2002, 43, 280–283. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Hou, X.; Wang, F.; Tang, C. Preparation and properties of zirconia nanotube-supported zirconium sulfate catalyst. React. Kinet. Mech. Catal. 2011, 104, 227–234. [Google Scholar] [CrossRef]
- Lamba, R.; Sarkar, S. Synergistic effect of mixed alcohols on esterification of decanoic acid with amberlyst 15 as catalyst. Environ. Prog. Sustain. Energy 2019, 38, 13103. [Google Scholar] [CrossRef]
- Shahid, A.; Jamal, Y.; Khan, S.J.; Khan, J.A.; Boulanger, B. Esterification Reaction Kinetics of Acetic and Oleic Acids with Ethanol in the Presence of Amberlyst 15. Arab. J. Sci. Eng. 2018, 43, 5701–5709. [Google Scholar] [CrossRef]
- Van Chuc, N.; Ngoc Quynh, B.; Mascunan, P.; Thi Thu Ha, V.; Fongarland, P.; Essayem, N. Esterification of aqueous lactic acid solutions with ethanol using carbon solid acid catalysts: Amberlyst 15, sulfonated pyrolyzed wood and graphene oxide. Appl. Catal. Gen. 2018, 552, 184–191. [Google Scholar]
- Gomes, G.J.; Fernanda Zalazar, M.; Arroyo, P.A.; Scremin, F.R.; Costa, M.B.; Bittencourt, P.R.S.; Lindino, C.A.; Peruchena, N.M. Molecular-level Understanding of the Rate-determining Step in Esterification Reactions Catalyzed by H-ZSM-5 Zeolite. An Experimental and Theoretical Study. Chemistryselect 2019, 4, 3031–3041. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Bokade, V.V. Esterification of Renewable Levulinic Acid to n-Butyl Levulinate over Modified H-ZSM-5. Chem. Eng. Technol. 2015, 38, 246–252. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Niphadkar, P.S.; Deshpande, S.S.; Bokade, V.V. Esterification of renewable levulinic acid to ethyl levulinate biodiesel catalyzed by highly active and reusable desilicated H-ZSM-5. J. Chem. Technol. Biotechnol. 2014, 89, 1507–1515. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Srinivasan, V.V.; Karvembu, R.; Luque, R. Preparation of mesoporous stannosilicates SnTUD-1 and catalytic activity in levulinic acid esterification. Microporous Mesoporous Mater. 2019, 287, 159–166. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, H.; Zhou, A.; Yang, F.; Shu, L.; She, Y.; Li, J.-R. Highly Efficient Catalytic Esterification in an -SO3H-Functionalized Cr(III)-MOF. Ind. Eng. Chem. Res. 2018, 57, 8388–8395. [Google Scholar] [CrossRef]
- Tarakci, M.I.S.; Ilgen, O. Esterification of Oleic Acid with Methanol Using Zr(SO4)2 as a Heterogeneous Catalyst. Chem. Eng. Technol. 2018, 41, 845–852. [Google Scholar] [CrossRef]
- Juan, J.C.; Zhang, J.; Yarmo, M.A. Study of catalysts comprising zirconium sulfate supported on a mesoporous molecular sieve HMS for esterification of fatty acids under solvent-free condition. Appl. Catal. Gen. 2008, 347, 133–141. [Google Scholar] [CrossRef]
- Juan, J.C.; Jiang, Y.; Meng, X.; Cao, W.; Yarmo, M.A.; Zhang, J. Supported zirconium sulfate on carbon nanotubes as water-tolerant solid acid catalyst. Mater. Res. Bull. 2007, 42, 1278–1285. [Google Scholar] [CrossRef]
- Jimenez-Morales, I.; Santamaria-Gonzalez, J.; Maireles-Torres, P.; Jimenez-Lopez, A. Calcined zirconium sulfate supported on MCM-41 silica as acid catalyst for ethanolysis of sunflower oil. Appl. Catal. B Environ. 2011, 103, 91–98. [Google Scholar] [CrossRef]
- Sohn, J.R.; Seo, D.H. Preparation of new solid superacid catalyst, zirconium sulfate supported on gamma-alumina and activity for acid catalysis. Catal. Today 2003, 87, 219–226. [Google Scholar] [CrossRef]
- Lavrenov, A.V.; Perelevskii, E.V.; Finevich, V.P.; Zaikovskii, V.I.; Paukshtis, E.A.; Duplyakin, V.K.; Bal’zhinimaev, B.S. Alkylation of isobutane with butenes on zirconium sulfate catalysts. Russ. J. Appl. Chem. 2003, 76, 550–557. [Google Scholar] [CrossRef]
- Tan, J.; Lu, T.; Zhang, J.; Xie, B.; Chen, M.; Zhu, X. Highly efficient and recyclable catalysts SnCl2-xH3PW10O40/AC with Bronsted and Lewis acid sites for terephthalic acid esterification. J. Taiwan Inst. Chem. Eng. 2018, 86, 18–24. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Bronsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Bronsted and Lewis Acid Sites on Zeolite for Glycerol Dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared-absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Arata, K.; Hino, M.; Yamagata, N. Acidity and catalytic activity of zirconium and titanium sulfates heat-treated at high-temperature solid surperacid catalysts. Bull. Chem. Soc. Jpn. 1990, 63, 244–246. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Bensalem, A.; Bozonverduraz, F.; Delamar, M.; Bugli, G. Preparation and characterization of highly dispersed silica-supported ceria. Appl. Catal. Gen. 1995, 121, 81–93. [Google Scholar] [CrossRef]
- Reddy, B.A.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.C. Surface characterization of CeO2/SiO2 and V2O5/CeO2/SiO2 catalysts by Raman, XPS, and other techniques. J. Phys. Chem. B 2002, 106, 10964–10972. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. Chemsuschem 2009, 2, 278–300. [Google Scholar] [CrossRef]







| Sample | Zr(SO4)2 Loading (wt.%) | BET Surface Area (m2/g) | Pore Volume (mL/g) | Average Pore Size (nm) | ||
|---|---|---|---|---|---|---|
| Vmicro | Vmeso | Dmicro | Dmeso | |||
| SiO2 | - | 214 | - | 1.40 | - | 26.8 |
| Zr(SO4)2/SiO2 | 28.5 | 139 | - | 0.95 | - | 22.5 |
| AC | - | 1053 | 0.34 | 0.21 | 1.39 | 14.8 |
| Zr(SO4)2/AC | 27.9 | 468 | 0.33 | 0.11 | 1.19 | 7.8 |
| Catalyst | B Acid Sites (mmol/g) | L Acid Sites (mmol/g) |
|---|---|---|
| Zr(SO4)2∙4H2O | 0.2011 | 0.0251 |
| Zr(SO4)2/SiO2 | 0.4182 | 0.0103 |
| Zr(SO4)2/AC | 0.3057 | 0.0012 |
| Sample | BEZr3d3/2 (eV) | BEZr3d5/2 (eV) | BEO1s (eV) |
|---|---|---|---|
| Zr(SO4)2∙4H2O | 186.9 | 184.5 | 532.6 |
| Zr(SO4)2/SiO2 | 186.6 | 184.2 | 532.9 |
| Zr(SO4)2/AC | 186.6 | 184.2 | 533.1 |
| SiO2 | - | - | 532.5 |
| AC | - | - | 532.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Chen, C.; Li, G.; Wang, Z.; Li, X. Active, Selective, and Recyclable Zr(SO4)2/SiO2 and Zr(SO4)2/Activated Carbon Solid Acid Catalysts for Esterification of Malic Acid to Dimethyl Malate. Catalysts 2020, 10, 384. https://doi.org/10.3390/catal10040384
Yu P, Chen C, Li G, Wang Z, Li X. Active, Selective, and Recyclable Zr(SO4)2/SiO2 and Zr(SO4)2/Activated Carbon Solid Acid Catalysts for Esterification of Malic Acid to Dimethyl Malate. Catalysts. 2020; 10(4):384. https://doi.org/10.3390/catal10040384
Chicago/Turabian StyleYu, Pei, Can Chen, Guangci Li, Zhong Wang, and Xuebing Li. 2020. "Active, Selective, and Recyclable Zr(SO4)2/SiO2 and Zr(SO4)2/Activated Carbon Solid Acid Catalysts for Esterification of Malic Acid to Dimethyl Malate" Catalysts 10, no. 4: 384. https://doi.org/10.3390/catal10040384