On the Exceptionally High Loading of L-Proline on Multi-Wall Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
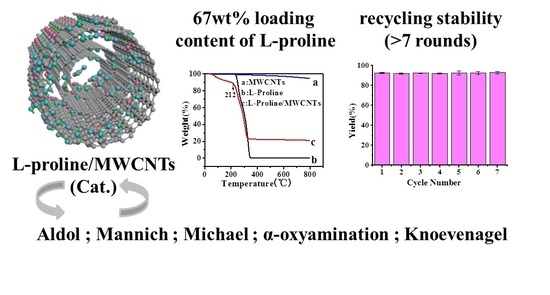
2.1. Characterization of the L-proline/MWCNTs Catalysts
2.2. Control Experiments
2.3. Application of the L-proline/MWCNTs Catalysts
3. Materials and Methods
3.1. Materials
3.2. Equipments
3.3. Typical Procedure for Catalyst Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamashita, Y.; Yasukawa, T.; Yoo, W.J.; Kitanosono, T.; Kobayashi, S. Catalytic Enantioselective Aldol Reactions. Chem. Soc. Rev. 2018, 47, 4388–4480. [Google Scholar] [PubMed]
- Yadav, G.D.; Singh, S. (L)-Prolinamide Imidazolium Hexafluorophosphate Ionic Liquid as an Efficient Reusable Organocatalyst for Direct Asymmetric Aldol Reaction in Solvent-Free Condition. RSC Adv. 2016, 6, 100459–100466. [Google Scholar]
- Wang, S.; Liu, P.; Wang, W.J.; Zhang, Z.; Li, B.G. Hyperbranched Polyethylene-Supported l-Proline: A Highly Selective and Recyclable Organocatalyst for Asymmetric Aldol Reactions. Catal. Sci. Technol. 2015, 5, 3798–3805. [Google Scholar]
- Ferré, M.; Pleixats, R.; Wong Chi Man, M.; Cattoën, X. Recyclable Organocatalysts Based on Hybrid Silicas. Green Chem. 2016, 18, 881–922. [Google Scholar]
- Hernández, J.G.; Juaristi, E. Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar]
- Chen, Q.; Xin, C.; Lou, L.L.; Yu, K.; Ding, F.; Liu, S. Polyoxometalate Supported Cinchona-Derived Chiral Amine for Asymmetric Organocatalysed Aldol Reaction. J. Inorg. Organomet. Polym. Mater. 2013, 23, 467–471. [Google Scholar]
- Das, D.; Pathak, G.; Rokhum, L. Polymer Supported DMAP: An Easily Recyclable Organocatalyst for Highly Atom-Economical Henry Reaction under Solvent-Free Conditions. RSC Adv. 2016, 6, 104154–104163. [Google Scholar]
- Liu, G.; Gu, H.; Sun, Y.; Long, J.; Xu, Y.; Li, H. Magnetically Recoverable Nanoparticles: Highly Efficient Catalysts for Asymmetric Transfer Hydrogenation of Aromatic Ketones in Aqueous Medium. Adv. Synth. Catal. 2011, 353, 1317–1324. [Google Scholar]
- Bortolini, O.; Caciolli, L.; Cavazzini, A.; Costa, V.; Greco, R.; Massi, A.; Pasti, L. Silica-Supported 5-(Pyrrolidin-2-Yl)Tetrazole: Development of Organocatalytic Processes from Batch to Continuous-Flow Conditions. Green Chem. 2012, 14, 992–1000. [Google Scholar]
- Bürgi, T.; Baiker, A. Heterogeneous Enantioselective Hydrogenation over Cinchona Alkaloid Modified Platinum: Mechanistic Insights into a Complex Reaction. Acc. Chem. Res. 2004, 37, 909–917. [Google Scholar]
- Maleki, A.; Firouzi-Haji, R. L-Proline Functionalized Magnetic Nanoparticles: A Novel Magnetically Reusable Nanocatalyst for One-Pot Synthesis of 2,4,6-Triarylpyridines. Sci. Rep. 2018, 8, 17303. [Google Scholar]
- Sóti, P.L.; Yamashita, H.; Sato, K.; Narumi, T.; Toda, M.; Watanabe, N.; Marosi, G.; Mase, N. Synthesis of a Self-Assembling Gold Nanoparticle-Supported Organocatalyst for Enamine-Based Asymmetric Aldol Reactions. Tetrahedron 2016, 72, 1984–1990. [Google Scholar]
- Abu-Reziq, R.; Alper, H.; Wang, D.; Post, M.L. Metal Supported on Dendronized Magnetic Nanoparticles: Highly Selective Hydroformylation Catalysts. J. Am. Chem. Soc. 2006, 128, 5279–5282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yu, H.; Tu, D.; Shen, L. L-Proline Functionalized Metal-Organic Framework PCN-261 as Catalyst for Aldol Reaction. Inorg. Chem. Commun. 2019, 107, 107448–107452. [Google Scholar]
- An, Z.; Guo, Y.; Zhao, L.; Li, Z.; He, J. L-Proline-Grafted Mesoporous Silica with Alternating Hydrophobic and Hydrophilic Blocks to Promote Direct Asymmetric Aldol and Knoevenagel-Michael Cascade Reactions. ACS Catal. 2014, 4, 2566–2576. [Google Scholar]
- Zayas, H.A.; Lu, A.; Valade, D.; Amir, F.; Jia, Z.; O’Reilly, R.K.; Monteiro, M.J. Thermoresponsive Polymer-Supported l-Proline Micelle Catalysts for the Direct Asymmetric Aldol Reaction in Water. ACS Macro Lett. 2013, 2, 327–331. [Google Scholar]
- Liu, Y.X.; Sun, Y.N.; Tan, H.H.; Tao, J.C. Asymmetric Aldol Reaction Catalyzed by New Recyclable Polystyrene-Supported l-Proline in the Presence of Water. Catal. Lett. 2008, 120, 281–287. [Google Scholar]
- Lu, J.; Toy, P.H. Organic Polymer Supports for Synthesis and for Reagent and Catalyst Immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar]
- An, Z.; Zhang, W.; Shi, H.; He, J. An effective heterogeneous L-proline catalyst for the asymmetric aldol reaction using anionic clays as intercalated support. J. Catal. 2006, 241, 319–327. [Google Scholar]
- Prasetyanto, E.A.; Lee, S.C.; Jeong, S.M.; Park, S.E. Chiral enhancement in diethyl malonate addition by morphosynthesized L-proline mesoporous silica. Chem. Comm. 2008, 17, 1995–1997. [Google Scholar]
- Safaei-ghomi, J.; Masoomi, R. Magnetic nanoscale core–shell structured Fe3O4@L-proline: An efficient, reusable and eco-friendly nanocatalyst for diastereoselective synthesis of fulleropyrrolidines. N. J. Chem. 2016, 40, 3289–3299. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Zahedi, S. L-Proline-Functionalized Fe3O4 Nanoparticles as a Novel Magnetic Chiral Catalyst for the Direct Asymmetric Mannich Reaction. Appl. Organomet. Chem. 2015, 29, 566–571. [Google Scholar]
- Chandrasekaran, S. Proline and benzylpenicillin derivatives grafted into mesoporous MCM-41: Novel organic–inorganic hybrid catalysts for direct aldol reaction. Indian Acad. Sci. 2003, 115, 365–372. [Google Scholar]
- John, J.; Gravel, E.; Namboothiri, I.N.N.; Doris, E. Advances in Carbon Nanotube-Noble Metal Catalyzed Organic Transformations. Nanotech. Rev. 2012, 1, 515–539. [Google Scholar] [CrossRef]
- Desmecht, A.; Pennetreau, F.; L’hoost, A.; Nircha, I.; Pichon, B.P.; Riant, O.; Hermans, S. Preparation of Magnetically Recoverable Carbon Nanotube-Supported Pd(II) Catalyst. Catal. Today 2019, 334, 24–29. [Google Scholar] [CrossRef]
- Sairanen, E.; Karinen, R.; Borghei, M.; Kauppinen, E.I.; Lehtonen, J. Preparation Methods for Multi-Walled Carbon Nanotube Supported Palladium Catalysts. ChemCatChem 2012, 4, 2055–2061. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z. Application of Immobilized Proline on CNTs and Proline Ionic Liquid as Novel Organocatalysts in the Synthesis of 2-Amino-4H-Pyran Derivatives: A Comparative Study between Their Catalytic Activities. ChemistrySelect 2017, 2, 8976–8982. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Kokotos, C.G.; Karousis, N.; Kokotos, G.; Tagmatarchis, N. Functionalized Multi-Walled Carbon Nanotubes in an Aldol Reaction. Nanoscale 2015, 7, 2750–2757. [Google Scholar] [CrossRef]
- Khoshnevis, M.; Eshghi, H. Diastereoselective Mannich Reaction with Prolinated MWCNTs as a Heterogeneous Organo-Nanocatalyst. J. Chem. Sci. 2020, 132, 41–51. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.; Tu, D.; Zhuang, Y.; Shen, L. L-Proline Functionalized Pillar-Layered MOF as a Heterogeneous Catalyst for Aldol Addition Reaction. Inorg. Chem. Comm. 2020, 119, 108052–108055. [Google Scholar]
- Khiar, N.; Navas, R.; Elhalem, E.; Valdivia, V.; Fernández, I. Proline-coated gold nanoparticles as a highly efficient nanocatalyst for the enantioselective direct aldol reaction in water. RSC Adv. 2013, 3, 3861–3864. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M.K.; Kumar, M. L-Proline Catalysed Multicomponent Synthesis of 3-Amino Alkylated Indoles via a Mannich-Type Reaction under Solvent-Free Conditions. Green Chem. 2012, 14, 290–295. [Google Scholar]
- Castán, A.; Badorrey, R.; Gálvez, J.A.; López-Ram-De-Víu, P.; Díaz-De-Villegas, M.D. Michael Addition of Carbonyl Compounds to Nitroolefins under the Catalysis of New Pyrrolidine-Based Bifunctional Organocatalysts. Org. Biomol. Chem. 2018, 16, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.P.; Brochu, M.P.; Sinz, C.J.; Macmillan, D.W.C. The Direct and Enantioselective Organocatalytic r-Oxidation of Aldehydes. J. Am. Chem. Soc. 2003, 125, 10808–10809. [Google Scholar]
- Momiyama, N.; Yamamoto, H. Catalytic Enantioselective Synthesis of α-Aminooxy and α-Hydroxy Ketone Using Nitrosobenzene. J. Am. Chem. Soc. 2003, 125, 6038–6039. [Google Scholar]
- Vaid, R.; Gupta, M. Silica-l-Proline: An Efficient and Recyclable Heterogeneous Catalyst for the Knoevenagel Condensation between Aldehydes and Malononitrile in Liquid Phase. Monats. Chem. 2015, 146, 645–652. [Google Scholar] [CrossRef]
- Karade, N.N.; Gampawar, S.V.; Shinde, S.V.B.; Jadhav, W.N. L-Proline Catalyzed Solvent-Free Knoevenagel Condensation for the Synthesis of 3-Substituted Coumarins. Chin. J. Chem. 2007, 25, 1686–1689. [Google Scholar] [CrossRef]
- Tan, R.; Li, C.; Luo, J.; Kong, Y.; Zheng, W.; Yin, D. An Effective Heterogeneous L-Proline Catalyst for the Direct Asymmetric Aldol Reaction Using Graphene Oxide as Support. J. Catal. 2013, 298, 138–147. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Reddy, N.R.; Sultana, S.S.; Narsihmulu, C.; Reddy, K.V. L-Proline catalysed asymmetric aldol reactions in PEG-400 as recyclable medium and transfer aldol reactions. Tetrahedron 2006, 62, 338–345. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Zhang, L.W.; Wu, L.Y.; Zhong, X.; Li, R.; Ma, J.T. Silica-supported pyrrolidine–triazole, an insoluble, recyclable organocatalyst for the enantioselective Michael addition of ketones to nitroalkenes. Tetrahedron Asymmetry 2008, 19, 1352–1355. [Google Scholar]
- Zhang, H.; Han, M.; Chen, T.; Xu, L.; Yu, L. Poly(N-isopropylacrylamide-co-L-proline)- catalyzed Claisen–Schmidt and Knoevenagel condensations: Unexpected enhanced catalytic activity of the polymer catalyst. RSC Adv. 2017, 1, 48214–48221. [Google Scholar] [CrossRef] [Green Version]
- Prabhakara, M.D.; Maiti, B. Ionic Liquid-Immobilized Proline(s) Organocatalyst-Catalyzed One-Pot Multi-Component Mannich Reaction under Solvent-Free Condition. Res. Chem. Intermediat. 2020, 46, 2381–2401. [Google Scholar] [CrossRef]
- Zhuo, C.; Xian, D.; Jian-wei, W.; Hui, X. An Efficient and Recyclable Ionic Liquid-Supported Proline Catalyzed Knoevenagel Condensation. ISRN Org. Chem. 2011, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Font, D.; Bastero, A.; Sayalero, S.; Jimeno, C.; Pericàs, M.A. Highly Enantioselective α-Aminoxylation of Aldehydes and Ketones with a Polymer-Supported Organocatalyst. Org. Lett. 2007, 9, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Omar, E.M.; Rahman, M.B.A.; Ni, B.; Headley, A.D. The Role of Neutral Anions in Ionic Liquid as Solvent Media for the Reactivity and Stereoselectivity towards Asymmetric Michael Addition Reaction of N-Pentanal with β-Nitrostyrene Catalyzed by L-Proline. Synth. Commun. 2019, 49, 1578–1591. [Google Scholar] [CrossRef]






| Nanocarbon | Yield (%) 1 in 1st Round | Yield (%) 1 in (Rounds Number) |
|---|---|---|
| C60 | trace | not available |
| Graphene oxide | 90 | 91 (7) |
| MWCNTs | 91 | 90 (7) |
| SWCNTs | 92 | 91 (7) |
| Activated carbon | 91 | 5 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liang, J.; Huang, S.; Yang, G.; Tian, K.; Chen, R.; Chen, H.; Zhang, Y. On the Exceptionally High Loading of L-Proline on Multi-Wall Carbon Nanotubes. Catalysts 2020, 10, 1246. https://doi.org/10.3390/catal10111246
Xu J, Liang J, Huang S, Yang G, Tian K, Chen R, Chen H, Zhang Y. On the Exceptionally High Loading of L-Proline on Multi-Wall Carbon Nanotubes. Catalysts. 2020; 10(11):1246. https://doi.org/10.3390/catal10111246
Chicago/Turabian StyleXu, Jiafang, Jichao Liang, Sheng Huang, Ge Yang, Keyi Tian, Ruonan Chen, Hongyu Chen, and Yanhua Zhang. 2020. "On the Exceptionally High Loading of L-Proline on Multi-Wall Carbon Nanotubes" Catalysts 10, no. 11: 1246. https://doi.org/10.3390/catal10111246