Author Contributions
Conceptualization, M.H. and A.A.A.; Methodology, M.H., S.K. and A.A.A.; Investigation, M.H., S.K., R.G. and N.R.; Resources, S.M., M.Z., S.A.A.S., Z.A.Z. and A.B.; Writing—Original Draft Preparation, M.H., A.A.A. and S.K. Writing—Review and Editing, A.A.A., S.M., N.R. and R.G.; Funding Acquisition, A.A.A. and Z.A.Z. Project Administration, A.A.A. and Z.A.Z. All authors have read and agreed to the published version of the manuscript.
Figure 1.
PXRD pattern of nanomaterials (MH1–MH5). (Powder patterns are matched with standard patterns—i.e., α-MnO2 ((JCPD No.44-0141), (JCPD No. 01-1127), CrO3 (JCPD No. 74-0326, 70-3766)).
Figure 1.
PXRD pattern of nanomaterials (MH1–MH5). (Powder patterns are matched with standard patterns—i.e., α-MnO2 ((JCPD No.44-0141), (JCPD No. 01-1127), CrO3 (JCPD No. 74-0326, 70-3766)).
Figure 2.
SEM images of the representative sample MH5 ((A) (100 nm) and (B) (400 nm)).
Figure 2.
SEM images of the representative sample MH5 ((A) (100 nm) and (B) (400 nm)).
Figure 3.
Photoluminescence (PL) spectra of the nanomaterials (MH–MH5).
Figure 3.
Photoluminescence (PL) spectra of the nanomaterials (MH–MH5).
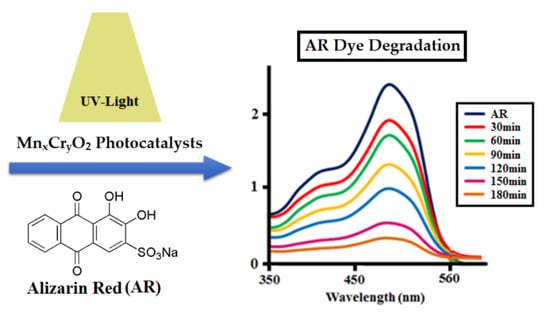
Figure 4.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) at different time intervals by 0.025 g of MH1 and MH5.
Figure 4.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) at different time intervals by 0.025 g of MH1 and MH5.
Figure 5.
Correlation between time and percentage degradation of alizarin red (AR) dye (250 ppm) by 0.025 g of nanomaterials MH1 and MH5.
Figure 5.
Correlation between time and percentage degradation of alizarin red (AR) dye (250 ppm) by 0.025 g of nanomaterials MH1 and MH5.
Figure 6.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) at different catalyst doses of MH4 and MH5 after 60 min of photocatalytic activity.
Figure 6.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) at different catalyst doses of MH4 and MH5 after 60 min of photocatalytic activity.
Figure 7.
Correlation between different catalyst doses of MH4 and MH5 and percentage degradation of alizarin red (AR) Dye (250 ppm) after 60 min of photocatalytic activity.
Figure 7.
Correlation between different catalyst doses of MH4 and MH5 and percentage degradation of alizarin red (AR) Dye (250 ppm) after 60 min of photocatalytic activity.
Figure 8.
Photocatalytic degradation of alizarin red (AR) dye at different dye concentrations (50–250 ppm) by 0.025 g of MH3 and MH5 after 60 min of photocatalytic activity.
Figure 8.
Photocatalytic degradation of alizarin red (AR) dye at different dye concentrations (50–250 ppm) by 0.025 g of MH3 and MH5 after 60 min of photocatalytic activity.
Figure 9.
Correlation between different dye concentrations (50–250 ppm) and percentage degradation of alizarin red (AR) dye by 0.025 g of nanomaterials MH1 and MH5 after 60 min of photocatalytic activity.
Figure 9.
Correlation between different dye concentrations (50–250 ppm) and percentage degradation of alizarin red (AR) dye by 0.025 g of nanomaterials MH1 and MH5 after 60 min of photocatalytic activity.
Figure 10.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) after 60 min employing different pH by 0.025 g of MH1 and MH5.
Figure 10.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) after 60 min employing different pH by 0.025 g of MH1 and MH5.
Figure 11.
Correlation between different pH and percentage degradation of alizarin red (AR) dye (200 ppm) by 0.02 g of nanomaterials MH1 and MH5 after 60 min of photocatalytic activity.
Figure 11.
Correlation between different pH and percentage degradation of alizarin red (AR) dye (200 ppm) by 0.02 g of nanomaterials MH1 and MH5 after 60 min of photocatalytic activity.
Figure 12.
Pseudo second order kinetics of alizarin red (AR) dye degradation at different time intervals by nanomaterials (MH1–MH5).
Figure 12.
Pseudo second order kinetics of alizarin red (AR) dye degradation at different time intervals by nanomaterials (MH1–MH5).
Figure 13.
Proposed mechanism of AR dye degradation over surface of catalyst.
Figure 13.
Proposed mechanism of AR dye degradation over surface of catalyst.
Table 1.
Synthetic conditions, powder X-ray diffraction (PXRD) peaks and found composition (energy dispersive X-ray; EDX) of synthesized nanomaterials (MH1–MH5).
Table 1.
Synthetic conditions, powder X-ray diffraction (PXRD) peaks and found composition (energy dispersive X-ray; EDX) of synthesized nanomaterials (MH1–MH5).
| Samples | Found Unit Formula | Synthetic Conditions | PXRD Peaks (2θ) | Found Composition by EDX |
|---|
| MH1 | Mn0.99Cr0.1O2 | 0.948 g KMnO4, 0.24 g K2Cr2O7, 20mL HCl | 12.65, 17.89, 28.59, 37.33, 41.77, 49.59, 56.31, 59.87, 69.37 | Mn (54.69); Cr (6.18); O (39.09) |
| MH2 | Mn0.87Cr0.15O2 | 0.948 g KMnO4, 0.41 g K2Cr2O7, 20mL HCl | 12.61, 18.03, 28.71, 37.41, 41.79, 49.67, 59.93, 69.31 | Mn (48.05); Cr (8.26); O (43.68) |
| MH3 | Mn0.99Cr0.13O2 | 0.948 g KMnO4, 0.35 g K2Cr2O7, 20mL HCl | 12.67, 18.09, 28.77, 37.41, 41.85, 49.71, 56.29, 60.11, 69.33 | Mn (54.88); Cr (7.21); O (37.90) |
| MH4 | Mn0.96Cr0.09O2 | 0.948 g KMnO4, 0.18 g K2Cr2O7, 20mL HCl | 13.09, 18.39, 28.85, 37.73, 42.07,50.13, 56.67, 60.25, 70.03 | Mn (53.05); Cr (5.05); O (41.88) |
| MH5 | Mn0.92Cr0.12O2 | 0.948 g KMnO4, 0.30 g K2Cr2O7, 20mL HCl | 12.77, 18.05, 28.65, 37.45, 41.95, 49.79, 56.25, 60.13, 69.03 | Mn (50.69); Cr (6.41); O (42.88) |
Table 2.
Synthetic conditions, composition and PXRD parameters of nanomaterials (MH1–MH6).
Table 2.
Synthetic conditions, composition and PXRD parameters of nanomaterials (MH1–MH6).
| Samples | Found Composition | Average Crystallite Size D (nm) | Volume V = D3 | Dislocation Density × 10−3 (nm)−2 (δ) | Micro Strain (ε) |
|---|
| MH1 | Mn0.99Cr0.1O2 | 44.02 ± 8 | 82,780 | 5.95 × 10−4 | 0.035 |
| MH2 | Mn0.87Cr0.15O2 | 38.90 ± 6 | 62,205 | 6.98 × 10−4 | 0.038 |
| MH3 | Mn0.99Cr0.13O2 | 49.60 ± 5 | 126,625 | 4.19 × 10−4 | 0.027 |
| MH4 | Mn0.96Cr0.09O2 | 50.22 ± 7 | 135,584 | 4.20 × 10−4 | 0.02 |
| MH5 | Mn0.92Cr0.12O2 | 36.82 ± 3 | 49,576 | 7.60 × 10−4 | 0.035 |
Table 3.
Morphological characteristics by SEM analysis of nanomaterials (MH1–MH5).
Table 3.
Morphological characteristics by SEM analysis of nanomaterials (MH1–MH5).
| Samples | Material Nature | Dispersity | Structural Appearance |
|---|
| MH1 | Monocrystalline | Monodisperse | Elongated Rod-like Structures |
| MH2 | Monocrystalline | Monodisperse | Elongated Rod-like Structures |
| MH3 | Monocrystalline | Monodisperse | Elongated Rod-like Structures |
| MH4 | Monocrystalline | Monodisperse | Short Edged Rods |
| MH5 | Monocrystalline | Monodisperse | Elongated Rod-like Structures |
Table 4.
Brunauer–Emmett–Teller (BET) properties of synthesized nanomaterials (MH1–MH5).
Table 4.
Brunauer–Emmett–Teller (BET) properties of synthesized nanomaterials (MH1–MH5).
| BET Properties | Samples |
|---|
| MH1 | MH2 | MH3 | MH4 | MH 5 |
|---|
| Surface ARea (SBET) (m2/g) | 37.153 | 33.998 | 46.325 | 37.515 | 20.906 |
| Pore Volume (Vm) (cc/g) | 0.048 | 0.018 | 0.038 | 0.043 | 0.032 |
| Pore Width (nm) | 3.775 | 2.425 | 2.583 | 3.537 | 4.887 |
| Constant C | 6951.982 | 4.652 | 19.11 | 93.29 | 2090.61 |
Table 5.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) by MH1–MH5 (0.025 g) at different time intervals.
Table 5.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) by MH1–MH5 (0.025 g) at different time intervals.
| Time (min) | MH1 | MH2 | MH3 | MH4 | MH5 |
|---|
| 30 | 46.21 | 44.94 | 45.61 | 44.21 | 47.23 |
| 60 | 57.11 | 52.40 | 52.12 | 53.02 | 54.15 |
| 90 | 61.78 | 61.00 | 62.09 | 63.07 | 62.20 |
| 120 | 67.78 | 66.18 | 68.21 | 68.22 | 67.98 |
| 150 | 79.29 | 78.79 | 78.76 | 79.51 | 77.13 |
| 180 | 89.06 | 88.99 | 86.76 | 88.23 | 89.89 |
Table 6.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) by different catalytic dosage of MH1–MH5 after 60 min of photocatalytic activity.
Table 6.
Photocatalytic degradation of alizarin red (AR) dye (250 ppm) by different catalytic dosage of MH1–MH5 after 60 min of photocatalytic activity.
| Amounts (g) | MH1 | MH2 | MH3 | MH4 | MH5 |
|---|
| 0.020 | 38.99 | 39.23 | 38.32 | 40.22 | 41.77 |
| 0.025 | 57.11 | 52.40 | 52.12 | 53.02 | 54.15 |
| 0.030 | 67.32 | 68.23 | 66.21 | 67.88 | 68.54 |
| 0.035 | 70.86 | 69.54 | 69.86 | 70.55 | 71.87 |
| 0.040 | 81.76 | 82.87 | 81.36 | 83.78 | 84.34 |
Table 7.
Photocatalytic degradation of different concentrations (50–250 ppm) of alizarin red (AR) dye by MH1–MH5 (0.025 g) after 60 min of photocatalytic activity.
Table 7.
Photocatalytic degradation of different concentrations (50–250 ppm) of alizarin red (AR) dye by MH1–MH5 (0.025 g) after 60 min of photocatalytic activity.
| Dye Concentration (ppm) | MH1 | MH2 | MH3 | MH4 | MH5 |
|---|
| 50 | 82.12 | 83.09 | 83.87 | 83.43 | 83.54 |
| 100 | 77.47 | 77 | 79.22 | 78.87 | 78.12 |
| 150 | 70.47 | 71 | 71.12 | 70.45 | 72.22 |
| 200 | 63.21 | 65.03 | 65.83 | 64.22 | 64.08 |
| 250 | 57.11 | 52.40 | 52.12 | 53.02 | 54.95 |
Table 8.
Photocatalytic degradation of alizarin red (AR) dye (200 ppm) by MH1–MH5 (0.02 g) after 60 min of photocatalytic activity.
Table 8.
Photocatalytic degradation of alizarin red (AR) dye (200 ppm) by MH1–MH5 (0.02 g) after 60 min of photocatalytic activity.
| pH | MH 1 | MH 2 | MH 3 | MH 4 | MH 5 |
|---|
| 3 | 25.62 | 26.20 | 26.86 | 25.92 | 26.14 |
| 5 | 27.69 | 27.11 | 28.29 | 28.68 | 27.88 |
| 7 | 49.22 | 48.01 | 49.78 | 49.89 | 50.22 |
| 9 | 64.63 | 64.98 | 65.22 | 65.18 | 67.63 |
| 11 | 76.71 | 76.02 | 76.10 | 76.97 | 78.51 |
Table 9.
Correlation (R2 Values) between factor studied and AR dye concentration after 60 min of photocatalytic activity (R2 Values).
Table 9.
Correlation (R2 Values) between factor studied and AR dye concentration after 60 min of photocatalytic activity (R2 Values).
| Samples | Correlation between Factor Studied and AR Dye Concentration after 60 min of Photocatalytic Activity (R2 Values) |
|---|
| Time | Catalyst Dose | Dye Concentration | pH |
|---|
| MH1 | 0.959 | 0.969 | 0.932 | 0.973 |
| MH2 | 0.94 | 0.968 | 0.928 | 0.972 |
| MH3 | 0.932 | 0.953 | 0.960 | 0.971 |
| MH4 | 0.946 | 0.943 | 0.954 | 0.865 |
| MH | 0.986 | 0.924 | 0.988 | 0.869 |