Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics
Abstract
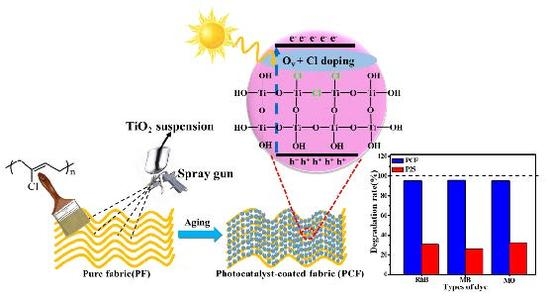
:1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Preparation of the TiO2 Suspension and Neoprene Adhesive
3.3. Preparation of Photocatalyst-Coated Fabric
3.4. Characterization
3.5. Photocatalytic Experiments
4. Conclusions
Supplementary materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Saad, S.A.; Isa, K.M.; Bahari, R. Chemically modified sugarcane bagasse as a potentially low-cost biosorbent for dye removal. Desalination 2010, 264, 123–128. [Google Scholar] [CrossRef]
- Aksu, Z. Biosorption of reactive dyes by dried activated sludge: Equilibrium and kinetic modelling. Biochem. Eng. J. 2001, 7, 79–84. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 2009, 15, 381–389. [Google Scholar] [CrossRef]
- Guptaa, V.K.; Guptaa, B.; Rastogi, A.; Agarwal, S.; Nayaka, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef]
- Phugare, S.S.; Kagalkar, A.N.; Govindwar, S.P.; Jadhav, J.P. A study on significant microbial interaction leading to decolorization and degradation of textile dye Rubine 3GP. J. Basic Microbiol. 2011, 51, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic TiO2 with Controlled Multiscale Porosity via a Template-Free Sol−Gel Process Accompanied by Phase Separation. Chem. Mater. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- In, S.L.; Orlov, A.D.; Berg, R.; Garcı, F.; Jimenez, S.P.; Tikhov, M.S.; Wright, D.; Lambert, R.M. Effective Visible Light-Activated B-Doped and B,N-Codoped TiO2 Photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.P.; Li, J.Z.; Wang, Q.; Zhou, W.; Tian, G.H.; Pan, K.; Tian, C.G.; Zou, J.L.; Fu, H.G. A Floating Porous Crystalline TiO2 Ceramic with Enhanced Photocatalytic Performance for Wastewater Decontamination. Eur. J. Inorg. Chem. 2013, 11, 2411–2417. [Google Scholar] [CrossRef]
- Liu, N.; Haublein, V.; Zhou, X.M.; Venkatesan, U.; Hartmann, M.; Mackovic, M.; Nakajima, T.; Spiecker, E.; Osvet, A.; Frey, L.; et al. “Black” TiO2 Nanotubes Formed by High-Energy Proton Implantation Show Noble-Metal-co-Catalyst Free Photocatalytic H2‑Evolution. Nano Lett. 2015, 15, 6815–6820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Feng, C.X.; Zhang, M.; Yang, J.J.; Zhang, Z.J. Visible light active N-doped TiO2 prepared from different precursors: Origin of the visible light absorption and photoactivity. Appl. Catal. B Environ. 2011, 104, 268–274. [Google Scholar] [CrossRef]
- Ren, W.J.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z.; Fan, X.X.; Zou, Z.G. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Yu, W.; Liu, X.J.; Pan, L.K.; Li, J.L.; Liu, J.Y.; Zhang, J.; Li, C.; Chen, P.; Sun, Z. Enhanced visible light photocatalytic degradation of methylene blue By F-doped TiO2. Appl. Surf. Sci. 2014, 319, 107–112. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.K.; Yu, J.G.; Yip, H.Y.; Wong, P.K.; Zhao, J.C. Efficient Visible-Light-Induced Photocatalytic Disinfection on Sulfur-Doped Nanocrystalline Titania. Environ. Sci. Technol. 2005, 394, 1175–1179. [Google Scholar] [CrossRef]
- Hong, X.T.; Wang, Z.P.; Cai, W.M.; Lu, F.; Zhang, J.; Yang, Y.Z.; Ma, N.; Liu, Y.J. Visible-Light-Activated Nanoparticle Photocatalyst of Iodine-Doped Titanium Dioxide. Chem. Mater. 2005, 17, 1548–1552. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.Y.; Lin, T.Q.; Yin, H.; Chen, P.; Wan, D.Y.; Xu, F.F.; Huang, F.Q.; Lin, J.H.; Xiand, X.M.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Xing, Z.P.; Zou, J.L.; Li, Z.Z.; Wu, X.Y.; Shen, L.Y.; Zhu, Q.; Yang, S.L.; Zhou, W. 3D urchin-like black TiO2−x/carbon nanotube heterostructures as efficient visible-light-driven photocatalysts. RSC Adv. 2017, 7, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Q.; Yang, C.Y.; Wang, Z.; Yin, H.; Lu, X.J.; Huang, F.Q.; Lin, J.H.; Xie, X.M.; Jiang, M.H. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 7, 967–972. [Google Scholar] [CrossRef]
- Yan, Y.B.; Miao, J.W.; Yang, Z.H.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y.H. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 32–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.C.; Zhao, D.; Ma, W.H.; Zhao, J.C. Change of Adsorption Modes of Dyes on Fluorinated TiO2 and Its Effect on Photocatalytic Degradation of Dyes under Visible Irradiation. Langmuir 2008, 24, 7338–7345. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.J.; Zhang, X.W.; Wang, L. Water-Mediated Promotion of Dye Sensitization of TiO2 under Visible Light. J. Am. Chem. Soc. 2011, 133, 10000–10002. [Google Scholar] [CrossRef]
- Choi, S.S.; Han, D.H. Recovery prediction of thermally aged chloroprene rubber composite using deformation test. J. Appl. Polym. Sci. 2008, 110, 3560–3565. [Google Scholar] [CrossRef]
- Kueseng, P.C.; Rattanasom, N.; Deeprasertkul, C. Improvement of the mechanical and thermal properties of silica-filled polychloroprene vulcanizates prepared from latex system. J. Appl. Polym. Sci. 2012, 124, 2657–2668. [Google Scholar]
- Berrk, K.; Liu, M.; Chakraborty, K.; Pullan, N.; West, A.; Sammon, C.; Topham, P. Mechanism for cross-linking polychloroprene with ethylene thiourea and zinc oxide. Rubber Chem. Technol. 2014, 88, 80–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Ding, X.; Chen, X.X.; Chen, Z.; Zheng, K.; Chen, L.; Ding, J.J.; Tian, X.Y.; Zhang, X. Layer-by-layer self-assembly photocatalytic nanocoatingon cotton fabrics as easily recycled photocatalyst for degrading gas and liquid pollutants. Cellulose 2017, 24, 4569–4580. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Preparation and Characterization of Quantum-Size Titanium Dioxide. J. Phys. Chem. 1988, 92, 5196–5201. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Z.; Zhang, L.Z.; Zhang, H.L.; Deng, F. Hierarchical chlorine-doped rutile TiO2 spherical clusters of nanorods: Large-scale synthesis and high photocatalytic activity. J. Solid State Chem. 2008, 181, 2516–2522. [Google Scholar] [CrossRef]
- Wang, X.K.; Wang, C.; Jiang, W.Q.; Guo, W.L.; Wang, J.G. Sonochemical synthesis and characterization of Cl-doped TiO2 and its application in the photodegradation of phthalate ester under visible light irradiation. Chem. Eng. J. 2012, 189–190, 288–294. [Google Scholar] [CrossRef]
- Hua, X.; Lizhi, Z. Selective Nonaqueous Synthesis of C-Cl-Codoped TiO2 with Visible-Light Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 11534–11541. [Google Scholar]
- Park, H.W.; Choi, W.Y. Effects of TiO2 Surface Fluorination on Photocatalytic Reactions and Photoelectrochemical Behaviors. J. Phys. Chem. B 2004, 108, 4086–4093. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.F.; Huang, R.; Zhang, Q.; Luo, S.L.; Au, C.T. Hollow peanut-like m-BiVO4: Facile synthesis and solar-light-induced photocatalytic property. CrystEngComm 2012, 14, 4217–4222. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Sato, T. Preparation and visible light induced photocatalytic activity of C-NaTaO3 and C-NaTaO3-Cl-TiO2 composite. Phys. Chem. Chem. Phys. 2013, 15, 20633–20640. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Huang, R.; Yin, S.F.; Luo, S.L.; Au, C.T. Porous peanut-like Bi2O3–BiVO4 composites with heterojunctions: One-step synthesis and their photocatalytic properties. Dalton Trans. 2012, 41, 9513–9518. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F- Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Shallow and deep trap emission and luminescence quenching of TiO2 nanoparticles on Cu doping. Appl. Nanosci. 2014, 4, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Gurylev, V.; Su, C.Y.; Perng, T.P. Surface reconstruction, oxygen vacancy distribution and photocatalytic activity of hydrogenated titanium oxide thin film. J. Catal. 2015, 330, 177–186. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, G.; Sun, J.X.; Rao, J.C.; Han, Z.H.; Hu, Y.D.; Xing, W.N.; Zhang, C.M. Doping effect of phosphate in Bi2WO6 and universal improved photocatalytic activity for removing various pollutants in water. Appl. Catal. B Environ. 2016, 188, 39–47. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Y.L.; Murowchick, J.; Chen, X.B. Vacuum-treated titanium dioxide nanocrystals: Optical properties, Surface disorder, oxygen vacancy, and photocatalytic activities. Catal. Today 2014, 225, 2–9. [Google Scholar] [CrossRef]
- Zhou, X.F.; Hu, C.; Hu, X.X.; Peng, T.W.; Qu, J.H. Plasmon-Assisted Degradation of Toxic Pollutants with Ag-AgBr/Al2O3 under Visible-Light Irradiation. J. Phys. Chem. C 2010, 114, 2746–2750. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhang, G.K.; Yu, S.J.; Shen, X. Efficient removal of organic contaminants by a visible light driven photocatalyst Sr6Bi2O9. Chem. Eng. J. 2010, 162, 171–177. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.S.; Yao, H.C.; Dang, L.Y.; Li, Z.J. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light activephotocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Zhang, T.; Ren, P.; Cao, D.; Lin, Y.; Wang, L.; Zhang, B.; Xiang, X. Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics. Catalysts 2020, 10, 69. https://doi.org/10.3390/catal10010069
Cao Z, Zhang T, Ren P, Cao D, Lin Y, Wang L, Zhang B, Xiang X. Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics. Catalysts. 2020; 10(1):69. https://doi.org/10.3390/catal10010069
Chicago/Turabian StyleCao, Zhen, Tingting Zhang, Pin Ren, Ding Cao, Yanjun Lin, Liren Wang, Bing Zhang, and Xu Xiang. 2020. "Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics" Catalysts 10, no. 1: 69. https://doi.org/10.3390/catal10010069