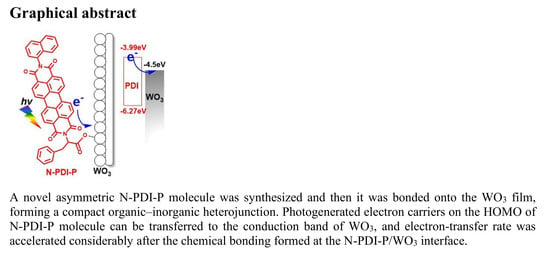
Chemically Bonded N-PDI-P/WO3 Organic-Inorganic Heterojunction with Improved Photoelectrochemical Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of N-PDI-P Molecule
 N and C
N and C  O bonded conjugation, which may weaken the reaction activity of carboxyl further. Hence, the bonding between carboxyl and inorganic semiconducting materials, such as WO3, is inhibited. The difference was that no conjugation occurred with at least one CH2 that was present between N-site and carboxyl. Theoretically, a prolonged C chain may increase the distance for electron transmission between PDI and inorganic semiconducting materials. As a result, PDI with one CH2 between N and carboxyl was synthesized in the present study.
O bonded conjugation, which may weaken the reaction activity of carboxyl further. Hence, the bonding between carboxyl and inorganic semiconducting materials, such as WO3, is inhibited. The difference was that no conjugation occurred with at least one CH2 that was present between N-site and carboxyl. Theoretically, a prolonged C chain may increase the distance for electron transmission between PDI and inorganic semiconducting materials. As a result, PDI with one CH2 between N and carboxyl was synthesized in the present study.2.2. Characterization of Intermolecular Bonding between Organic and Inorganic Molecules
 C
C  O in carboxylate, respectively [30]. This observation reflected that N-PDI-P bonded onto the WO3 surface successfully. According to Raman spectra (Figure 2g), N-PDI-P had a characteristic C–H adsorption peak at 2800 cm−1, a characteristic C–N adsorption peak at 2260 cm−1, a characteristic C=O adsorption peak on aromatic at 1620 cm−1, and a characteristic C–O–C adsorption peak on tert-butoxycarbonyl at 690 cm−1. WO3 nanofilm had evident adsorption peaks at 269, 713, and 806 cm−1, which belonged to its lattice vibration. However, the composite had a new peak at 940 cm−1, which showed the formation of a new bond, which may be caused by the vibration coupling of O
O in carboxylate, respectively [30]. This observation reflected that N-PDI-P bonded onto the WO3 surface successfully. According to Raman spectra (Figure 2g), N-PDI-P had a characteristic C–H adsorption peak at 2800 cm−1, a characteristic C–N adsorption peak at 2260 cm−1, a characteristic C=O adsorption peak on aromatic at 1620 cm−1, and a characteristic C–O–C adsorption peak on tert-butoxycarbonyl at 690 cm−1. WO3 nanofilm had evident adsorption peaks at 269, 713, and 806 cm−1, which belonged to its lattice vibration. However, the composite had a new peak at 940 cm−1, which showed the formation of a new bond, which may be caused by the vibration coupling of O  C
C  O of carboxylate. Hence, N-PDI-P is bonded onto the WO3 surface through carboxyl successfully. UV-vis diffuse reflectance (Figure 2h) showed that the λedge of WO3 edge was approximately 446 nm, and the composite produced a small red shift (approximately 455 nm). The adsorption peaks of N-PDI-P were in the range of 450–550 nm, which proved the improved absorbance of the composite. One shoulder peak with uncertain peak position was observed in the range of 510–530 nm, which corresponded to the peak of N-PDI-P at 525 nm. N-PDI-P had a characteristic peak at 489 nm, which produced a blue shift to 481 nm after bonding, thereby showing that solid organic molecules were formed by an H-aggregate [31].
O of carboxylate. Hence, N-PDI-P is bonded onto the WO3 surface through carboxyl successfully. UV-vis diffuse reflectance (Figure 2h) showed that the λedge of WO3 edge was approximately 446 nm, and the composite produced a small red shift (approximately 455 nm). The adsorption peaks of N-PDI-P were in the range of 450–550 nm, which proved the improved absorbance of the composite. One shoulder peak with uncertain peak position was observed in the range of 510–530 nm, which corresponded to the peak of N-PDI-P at 525 nm. N-PDI-P had a characteristic peak at 489 nm, which produced a blue shift to 481 nm after bonding, thereby showing that solid organic molecules were formed by an H-aggregate [31].2.3. Performance of N-PDI-P/WO3 Organic-Inorganic Heterojunction
2.4. Charge Transfer of N-PDI-P/WO3 Organic-Inorganic Interface
3. Materials and Methods
3.1. General Procedure for Synthesis of N-PDI-P
3.2. Preparation of N-PDI-P/WO3 Organic-Inorganic Heterojunction
3.3. Calculation, Characterization, and Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ouyang, T.; Ye, Y.-Q.; Wu, C.-Y.; Xiao, K.; Liu, Z.-Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and β-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef]
- Ge, J.; Yin, W.-J.; Yan, Y. Solution-Processed Nb-Substituted BaBiO3 Double Perovskite Thin Films for Photoelectrochemical Water Reduction. Chem. Mater. 2018, 30, 1017–1031. [Google Scholar] [CrossRef]
- Xu, S.; Fu, D.; Song, K.; Wang, L.; Yang, Z.; Yang, W.; Hou, H. One-dimensional WO3/BiVO4 heterojunction photoanodes for efficient photoelectrochemical water splitting. Chem. Eng. J. 2018, 349, 368–375. [Google Scholar] [CrossRef]
- Landman, A.; Dotan, H.; Shter, G.E.; Wullenkord, M.; Houaijia, A.; Maljusch, A.; Grader, G.S.; Rothschild, A. Photoelectrochemical water splitting in separate oxygen and hydrogen cells. Nat. Mater. 2017, 16, 646–651. [Google Scholar] [CrossRef]
- Klepser, B.M.; Bartlett, B.M. Anchoring a Molecular Iron Catalyst to Solar-Responsive WO3 Improves the Rate and Selectivity of Photoelectrochemical Water Oxidation. J. Am. Chem. Soc. 2014, 136, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Z.; Yin, X.; Kvit, A.; Liao, Q.; Kang, Z.; Yan, X.; Zhang, Y.; Wang, X. Enhanced photoelectrochemical efficiency and stability using a conformal TiO2 film on a black silicon photoanode. Nat. Energy 2017, 2, 17045. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Liu, Y.; Huang, C.; Liu, W.; Mi, X.; Fan, D.; Fan, F.; Lv, H.; Chen, X. SnS2 Nanosheets/H-TiO2 Nanotube Arrays as a Type II Heterojunctioned Photoanode for Photoelectrochemical Water Splitting. ChemSusChem 2019, 12, 958. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Chen, Y.; Qin, Z.; Wang, M.; Guo, L. Facile Fabrication of Sandwich Structured WO3 Nanoplate Arrays for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Inter. 2016, 8, 18089–18096. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Meng, D.; Wang, D.; Xie, T. Fabrication of metallic charge transfer channel between photoanode Ti/Fe2O3 and cocatalyst CoOx: An effective strategy for promoting photoelectrochemical water oxidation. J. Mater. Chem. A 2016, 4, 16661–16669. [Google Scholar] [CrossRef]
- Zheng, B.-F.; Ouyang, T.; Wang, Z.; Long, J.; Chen, Y.; Liu, Z.-Q. Enhanced plasmon-driven photoelectrocatalytic methanol oxidation on Au decorated α-Fe2O3 nanotube arrays. Chem. Commun. 2018, 54, 9583–9586. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ma, W.; Chen, J.-F.; Liu, X.-Y.; Peng, Y.-Y.; Yang, Z.-Y.; Tian, H.; Long, Y.-T. Quantifying visible-light-induced electron transfer properties of single dye-Sensitized ZnO entity for water splitting. J. Am. Chem. Soc. 2018, 140, 5272–5279. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhu, Q.; Zhang, M.; Yan, Y.; Liu, Y.; Li, M.; Yang, Z.; Geng, P. Synthesis of hexagonal ultrathin tungsten oxide nanowires with diameters below 5 nm for enhanced photocatalytic performance. Superlattice. Microst 2018, 116, 17–26. [Google Scholar]
- Hu, J.; Duan, W.; He, H.; Lv, H.; Huang, C.; Ma, X. A promising strategy to tune the Schottky barrier of a MoS2(1−x)Se2x/graphene heterostructure by asymmetric Se doping. J. Mater. Chem. C 2019, 7, 7798–7805. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Z.; Chen, D.; Zhou, M.; Wei, J. Flake-like NiO/WO3 p-n heterojunction photocathode for photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 440, 1101–1106. [Google Scholar] [CrossRef]
- Hua, X.; Ma, X.; Hu, J.; He, H.; Xu, G.; Huang, C.; Chen, X. Controlling electronic properties of MoS2/graphene oxide heterojunctions for enhancing photocatalytic performance: The role of oxygen. Phys. Chem. Chem. Phys. 2018, 20, 1974–1983. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Luo, Z.; Li, C.; Zhang, D.; Wang, T.; Gong, J. Highly-oriented Fe2O3/ZnFe2O4 nanocolumnar heterojunction with improved charge separation for photoelectrochemical water oxidation. Chem. Commun. 2016, 52, 9013–9015. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, B.; Wu, F.; Zhang, F.; Lu, J.E.; Kang, X.; Ping, Y.; Chen, S. Point of Anchor: Impacts on Interfacial Charge Transfer of Metal Oxide Nanoparticles. J. Am. Chem. Soc. 2018, 140, 15290–15299. [Google Scholar] [CrossRef]
- Bellani, S.; Ghadirzadeh, A.; Meda, L.; Savoini, A.; Tacca, A.; Marra, G.; Meira, R.; Morgado, J.; Di Fonzo, F.; Antognazza, M.R. Hybrid Organic/Inorganic Nanostructures for Highly Sensitive Photoelectrochemical Detection of Dissolved Oxygen in Aqueous Media. Adv. Funct. Mater. 2015, 25, 4531–4538. [Google Scholar] [CrossRef]
- Wu, Q.; Li, L.; Hai, J.; Zhang, X.; Lu, Z.; Yang, J.; Liu, Y.; Zhang, L.; Zhan, C. Edge-to-face stacking non-fullerene small molecule acceptor for bulk heterojunction solar cells. Dyes Pigment. 2016, 132, 41–47. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, B.; Zhang, X.; Tang, A.; Chen, L.; Zhan, C.; Yao, J. Perylene–Diimide Based Non-Fullerene Solar Cells with 4.34% Efficiency through Engineering Surface Donor/Acceptor Compositions. Chem. Mater. 2014, 26, 2907–2914. [Google Scholar] [CrossRef]
- Sun, T.; Song, J.; Jia, J.; Li, X.; Sun, X. Real roles of perylenetetracarboxylic diimide for enhancing photocatalytic H2-production. Nano Energy 2016, 26, 83–89. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Nanda, K.K.; Swain, S.; Satpati, B.; Besra, L.; Mishra, B.; Chaudhary, Y.S. Enhanced Photocatalytic Activity and Charge Carrier Dynamics of Hetero-Structured Organic–Inorganic Nano-Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 7970–7978. [Google Scholar] [CrossRef]
- He, J.; Hagfeldt, A.; Lindquist, S.-E.; Grennberg, H.; Korodi, F.; Sun, L.; Akermark, B. Phthalocyanine-Sensitized Nanostructured TiO2 Electrodes Prepared by a Novel Anchoring Method. Langmuir 2001, 17, 2743–2747. [Google Scholar] [CrossRef]
- Lu, Z.; Zhan, C.; Yu, X.; He, W.; Jia, H.; Chen, L.; Tang, A.; Huang, J.; Yao, J. Large-scale, ultra-dense and vertically standing zinc phthalocyanine π–π stacks as a hole-transporting layer on an ITO electrode. J. Mater. Chem. 2012, 22, 23492–23496. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, M.A.; Islam, M.A.; Hahn, J.R. Solar-Light-Driven Efficient ZnO–Single-Walled Carbon Nanotube Photocatalyst for the Degradation of a Persistent Water Pollutant Organic Dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Darensbourg, D.J.; Atnip, E.V.; Klausmeyer, K.K.; Reibenspies, J.H. Amino Acid Derivatives of Tungsten Carbonyl. Structure and Reactivity Investigations of Zerovalent Tungsten Glycine Derivatives. Inorg. Chem. 1994, 33, 5230–5237. [Google Scholar] [CrossRef]
- Yao, H.; Domoto, K.; Isohashi, T.; Kimura, K. In Situ Detection of Birefringent Mesoscopic H and J Aggregates of Thiacarbocyanine Dye in Solution. Langmuir 2005, 21, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Z.; Ye, L.; Zhan, C.; Hou, J.; Zhang, S.; Jiang, B.; Zhao, Y.; Huang, J.; Zhang, S.; et al. A Potential Perylene Diimide Dimer-Based Acceptor Material for Highly Efficient Solution-Processed Non-Fullerene Organic Solar Cells with 4.03% Efficiency. Adv. Mater. 2013, 25, 5791–5797. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yan, Y.; Zhang, M.; Tan, H.; Geng, P.; Le, S.; Yang, Z.; Liu, Y. The effects of adjusting pulse anodization parameters on the surface morphology and properties of a WO3 photoanode for photoelectrochemical water splitting. J. Solid State Electrochem. 2018, 22, 2169–2181. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Mi, X.; Zhong, D.; Zhang, W.; Liu, Y.; Fan, D.; Li, M.; Hai, J.; Lu, Z. Chemically Bonded N-PDI-P/WO3 Organic-Inorganic Heterojunction with Improved Photoelectrochemical Performance. Catalysts 2020, 10, 122. https://doi.org/10.3390/catal10010122
Feng C, Mi X, Zhong D, Zhang W, Liu Y, Fan D, Li M, Hai J, Lu Z. Chemically Bonded N-PDI-P/WO3 Organic-Inorganic Heterojunction with Improved Photoelectrochemical Performance. Catalysts. 2020; 10(1):122. https://doi.org/10.3390/catal10010122
Chicago/Turabian StyleFeng, Cheng, Xihong Mi, Dingwen Zhong, Weiming Zhang, Yongping Liu, Dayong Fan, Ming Li, Jiefeng Hai, and Zhenhuan Lu. 2020. "Chemically Bonded N-PDI-P/WO3 Organic-Inorganic Heterojunction with Improved Photoelectrochemical Performance" Catalysts 10, no. 1: 122. https://doi.org/10.3390/catal10010122