Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review †
Abstract
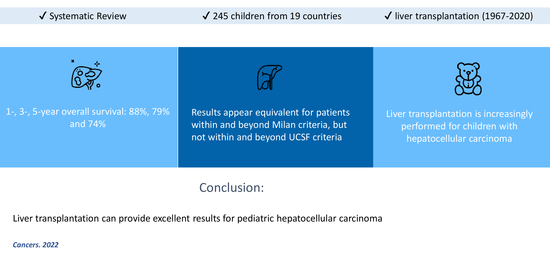
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Search Strategy and Eligibility Criteria
- Participants: Patients < 18 years of age of any sex or race undergoing LT for HCC
- Interventions: LT
- Comparison: Not applicable
- Outcomes: Overall Survival (OS) and disease-free survival (DFS)
- Study Design: randomized clinical trials or non-randomized (either prospective or retrospective) clinical studies, case series, or case reports.
2.2. Data Tabulation and Extraction
2.3. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Synthesis of Results
3.2.1. Complications
3.2.2. Disease-Free Survival
3.2.3. Overall Survival
3.2.4. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lau, C.S.; Mahendraraj, K.; Chamberlain, R.S. Hepatocellular Carcinoma in the Pediatric Population: A Population Based Clinical Outcomes Study Involving 257 Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1973–2011). HPB Surg. 2015, 2015, 670728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.W.; Millar, A.J.; Hadley, G.P.; Ionescu, G.; Kruger, M.; Poole, J.; Stones, D.; Wainright, L.; Chitnis, M.; Wessels, G. Hepatocellular carcinoma and liver tumors in South African children: A case for increased prevalence. Cancer 2004, 101, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, P.; Mackinlay, G.; Perilongo, G.; Brown, J.; Shafford, E.; Aronson, D.; Pritchard, J.; Chapchap, P.; Keeling, J.; Plaschkes, J.; et al. Hepatocellular carcinoma in children: Results of the first prospective study of the International Society of Pediatric Oncology group. J. Clin. Oncol. 2002, 20, 2798–2804. [Google Scholar] [CrossRef]
- Murawski, M.; Weeda, V.B.; Maibach, R.; Morland, B.; Roebuck, D.J.; Zimmerman, A.; Casanova, M.; Perilongo, G.; Laithier, V.; Kebudi, R.; et al. Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned From the SIOPEL 2 and 3 Studies. J. Clin. Oncol. 2016, 34, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Sharif, K.; Brown, R.M.; Morland, B. Hepatocellular carcinoma in children. Clin. Liver Dis. 2015, 19, 433–447. [Google Scholar] [CrossRef] [PubMed]
- de Ville de Goyet, J.; Meyers, R.L.; Tiao, G.M.; Morland, B. Beyond the Milan criteria for liver transplantation in children with hepatic tumours. Lancet Gastroenterol. Hepatol. 2017, 2, 456–462. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Benedetti, D.J.; Matsuoka, L.K.; Izzy, M.; Rauf, M.A.; Pai, A.K.; Bailey, C.E.; Alexopoulos, S.P. Surgical management of pediatric hepatocellular carcinoma: An analysis of the National Cancer Database. J. Pediatric Surg. 2021, 56, 772–777. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Ye, F.; Zhao, Z.; Matsuoka, L.K.; Montenovo, M.I.; Izzy, M.; Benedetti, D.J.; Lovorn, H.N., 3rd; Gillis, L.A.; Alexopoulos, S.P. Population-Based Analysis of Hepatocellular Carcinoma in Children: Identifying Optimal Surgical Treatment. J. Am. Coll. Surg. 2020, 230, 1035–1044. [Google Scholar] [CrossRef]
- Ismail, H.; Broniszczak, D.; Kaliciński, P.; Markiewicz-Kijewska, M.; Teisseyre, J.; Stefanowicz, M.; Szymczak, M.; Dembowska-Bagińska, B.; Kluge, P.; Perek, D.; et al. Liver transplantation in children with hepatocellular carcinoma Do Milan criteria apply to pediatric patients? Pediatric Transpl. 2009, 13, 682–692. [Google Scholar] [CrossRef]
- Pham, T.A.; Gallo, A.M.; Concepcion, W.; Esquivel, C.O.; Bonham, C.A. Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg. 2015, 150, 1150–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Deveraux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2017. [Google Scholar]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; Article No. 38. pp. 1–10. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bachetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.1 (updated September 2020); Cochrane: London, UK, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.r-project.org/ (accessed on 13 March 2021).
- D’Souza, A.M.; Towbin, A.J.; Gupta, A.; Alonso, M.; Nathan, J.D.; Bondoc, A.; Tiao, G.; Geller, J.I. Clinical heterogeneity of pediatric hepatocellular carcinoma. Pediatric Blood Cancer 2020, 67, e28307. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, Y.; Xia, L.; Qiu, B.J.; Zhou, T.; Feng, M.X.; Xue, F.; Chen, X.S.; Han, L.S.; Zhang, J.J.; et al. Living-donor liver transplantation for children with tyrosinemia type I. J. Dig. Dis. 2020, 21, 189–194. [Google Scholar] [CrossRef]
- Karaca, C.A.; Yilmaz, C.; Farajov, R.; Iakobadze, Z.; Aydogdu, S.; Kilic, M. Live donor liver transplantation for type 1 tyrosinemia: An analysis of 15 patients. Pediatric Transpl. 2019, 23, e13498. [Google Scholar] [CrossRef]
- Waich, S.; Roscher, A.; Brunner-Krainz, M.; Cortina, G.; Köstl, G.; Feichtinger, R.G.; Entenmann, A.; Müller, T.; Knisely, A.S.; Mayr, J.A.; et al. Severe Deoxyguanosine Kinase Deficiency in Austria: A 6-Patient Series. J. Pediatric Gastroenterol. Nutr. 2019, 68, e1–e6. [Google Scholar] [CrossRef]
- Valamparampil, J.J.; Reddy, M.S.; Shanmugam, N.; Vij, M.; Kanagavelu, R.G.; Rela, M. Living donor liver transplantation in Alagille syndrome-Single center experience from south Asia. Pediatric Transpl. 2019, 23, e13579. [Google Scholar] [CrossRef]
- Kumar, K.; Almanea, H.; Broering, D.; Shagrani, M. Early Hepatocellular Carcinoma Associated With Fibrocystic Liver Disease in a 10-Year-Old Child: A Case Report. Transpl. Proc. 2019, 51, 3147–3149. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Hong, S.A.; Oh, S.H.; Kim, K.M.; Yoo, H.W.; Kim, G.H.; Yu, E. Progressive Familial Intrahepatic Cholestasis in Korea: A Clinicopathological Study of Five Patients. J. Pathol. Transl. Med. 2019, 53, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Timothy, L.D.; Lehrke, H.D.; Chandan, V.S.; Kolbe, A.B.; Furuya, K.N. Diffuse Adenomatosis and Hepatocellular Carcinoma Treated with Liver Transplantation in an Adolescent Female with Kabuki Syndrome with a Novel KMT2D Gene Mutation. Case Rep. Pediatrics 2019, 2019, 7983824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiusanen, T.; Hukkinen, M.; Leskinen, O.; Soini, T.; Kanerva, J.A.; Jahnukainen, T.; Mäkisalo, H.; Heikinheimo, M.; Pakarinen, M.P. Incidence and long-term outcomes of surgically treated childhood hepatic malignancies in Finland. Acta Paediatr. 2020, 109, 404–414. [Google Scholar] [CrossRef]
- Cowell, E.; Patel, K.; Heczey, A.; Finegold, M.; Venkatramani, R.; Wu, H.; López-Terrada, D.; Miloh, T. Predisposing Conditions to Pediatric Hepatocellular Carcinoma and Association with Outcomes: Single-center Experience. J. Pediatric Gastroenterol. Nutr. 2019, 68, 695–699. [Google Scholar] [CrossRef]
- Chen, E.; Rangaswami, A.; Esquivel, C.O.; Concepcion, W.; Lungren, M.; Thakor, A.S.; Yoo, C.H.; Donaldson, S.S.; Hiniker, S.M. Orthotopic Liver Transplantation After Stereotactic Body Radiotherapy for Pediatric Hepatocellular Carcinoma with Central Biliary Obstruction and Nodal Involvement. Cureus 2018, 10, e3499. [Google Scholar] [CrossRef] [Green Version]
- Vinayak, R.; Cruz, R.J., Jr.; Ranganathan, S.; Mohanka, R.; Mazariegos, G.; Soltys, K.; Bond, G.; Tadros, S.; Humar, A.; Marsh, J.W. Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981–2015). Liver Transpl. 2017, 23, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, M.A.; Warad, D.M.; Matsumoto, J.M.; Heimbach, J.K.; El-Youssef, M.; Arndt, C.A.S.; Rodriguez, V.; Rao, A.A.N. Management of pediatric hepatocellular carcinoma: A multimodal approach. Pediatric Transpl. 2017, 21, e13007. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Brecklin, B.; Vachharajani, N.; Subramanian, V.; Nadler, M.; Stoll, J.; Turmelle, Y.; Lowell, J.A.; Chapman, W.C.; Doyle, M.M. Liver Transplantation for Malignant Primary Pediatric Hepatic Tumors. J. Am. Coll. Surg. 2017, 225, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Geramizadeh, B.; Kashkooe, A.; Bahador, A.; Dehgani, S.M.; Shamsaeefar, A.; Kazemi, K.; Malekhosseini, S.A. Pediatric hepatocellular carcinoma, a single center study from the South of Iran: Case series. Hepat. Mon. 2017, 17, e11837. [Google Scholar] [CrossRef] [Green Version]
- DE Pasquale, M.D.; de Ville de Goyet, J.; Monti, L.; Grimaldi, C.; Crocoli, A.; Castellano, A. Bevacizumab Combined with Chemotherapy in Children Affected by Hepatocellular Carcinoma: A Single-center Experience. Anticancer Res. 2017, 37, 1489–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troisi, R.I.; Van Huysse, J.; Berrevoet, F.; Vandenbossche, B.; Sainz-Barriga, M.; Vinci, A.; Ricciardi, S.; Bocchetti, T.; Rogiers, X.; de Hemptinne, B. Evolution of laparoscopic left lateral sectionectomy without the Pringle maneuver: Through resection of benign and malignant tumors to living liver donation. Surg. Endosc. 2011, 25, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Haberal, M.; Akdur, A.; Moray, G.; Arslan, G.; Özçay, F.; Selçuk, H.; Özdemir, H. Expanded Criteria for Hepatocellular Carcinoma in Liver Transplant. Exp. Clin. Transpl. 2017, 15 (Suppl. 2), 55–58. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, P.; Kogan-Liberman, D.; Thompson, J.F.; Schwartz, D.; Pan, D.H. Hepatocellular Carcinoma in a Child With Chronic Hepatitis C and α-1 Antitrypsin Heterozygosity. J. Pediatric Gastroenterol. Nutr. 2017, 64, e22–e24. [Google Scholar] [CrossRef]
- Benedict, M.; Rodriguez-Davalos, M.; Emre, S.; Walther, Z.; Morotti, R. Congenital Extrahepatic Portosystemic Shunt (Abernethy Malformation Type Ib) With Associated Hepatocellular Carcinoma: Case Report and Literature Review. Pediatric Dev. Pathol. 2017, 20, 354–362. [Google Scholar] [CrossRef]
- Friend, B.D.; Venick, R.S.; McDiarmid, S.V.; Zhou, X.; Naini, B.; Wang, H.; Farmer, D.G.; Busuttil, R.W.; Federman, N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatric Blood Cancer 2017, 64, e26682. [Google Scholar] [CrossRef]
- Imseis, E.M.; Bynon, J.S.; Thornhill, C. Case of hepatocellular carcinoma in a patient with hereditary tyrosinemia in the post-newborn screening era. World J. Hepatol. 2017, 9, 487–490. [Google Scholar] [CrossRef]
- Triana, P.; Dore, M.; Romo, M.M.; Gomez, J.J.; Galán, A.S.; Hernandez, F.; Moreno, A.M.A.; Encinas, J.L.; Martinez, L.; Santamaria, M.L. Hepatocellular Carcinoma: Referral to a Transplantation Unit. Eur. J. Pediatric Surg. 2017, 27, 16–19. [Google Scholar] [CrossRef]
- Shah, I.; Shah, F. Tyrosinemia type I: Case series with response to treatment to NTBC. Indian J. Gastroenterol. 2016, 35, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.; Borkar, V.V.; Safwan, M.; Vij, M.; Govil, S.; Shanmugam, N.; Rela, M. Pediatric hepatocellular carcinoma in a developing country: Is the etiology changing? Pediatric Transpl. 2016, 20, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ko, J.S.; Seo, J.K.; Moon, J.S.; Park, S.S. Clinical and ABCB11 profiles in Korean infants with progressive familial intrahepatic cholestasis. World J. Gastroenterol. 2016, 22, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- Picoraro, J.A.; Ovchinsky, N.; Martinez, M.; Lobritto, S.J.; Satwani, P.; Ramphal, R.; Cairo, M.S.; Kato, T. First Attempt of Sequential Living Donor Liver and Hematopoietic Stem Cell Transplantation in a Child With Advanced Hepatocellular Carcinoma: Case Report. Transpl. Proc. 2016, 48, 3174–3177. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, M.R.; Abaalkhail, F.; Al-Manea, H. Misdiagnosed or Incidentally Detected Hepatocellular Carcinoma in Explanted Livers: Lessons Learned. Ann. Transpl. 2015, 20, 366–372. [Google Scholar] [CrossRef]
- Yu, S.B.; Kim, H.Y.; Eo, H.; Won, J.K.; Jung, S.E.; Park, K.W.; Kim, W.K. Clinical characteristics and prognosis of pediatric hepatocellular carcinoma. World J. Surg. 2006, 30, 43–50. [Google Scholar] [CrossRef]
- Samuk, I.; Tekin, A.; Tryphonopoulos, P.; Pinto, I.G.; Garcia, J.; Weppler, D.; Levi, D.M.; Nishida, S.; Selvaggi, G.; Ruiz, P.; et al. Abdominal transplantation for unresectable tumors in children: The zooming out principle. Pediatric Surg. Int. 2016, 32, 337–346. [Google Scholar] [CrossRef]
- Seda Neto, J.; Leite, K.M.; Porta, A.; Fonseca, E.A.; Feier, F.H.; Pugliese, R.; Miura, I.K.; Chapchap, P.; Porta, G. HCC prevalence and histopathological findings in liver explants of patients with hereditary tyrosinemia type 1. Pediatric Blood Cancer 2014, 61, 1584–1589. [Google Scholar] [CrossRef]
- Bartlett, D.C.; Lloyd, C.; McKiernan, P.J.; Newsome, P.N. Early nitisinone treatment reduces the need for liver transplantation in children with tyrosinaemia type 1 and improves post-transplant renal function. J. Inherit. Metab. Dis. 2014, 37, 745–752. [Google Scholar] [CrossRef]
- Malik, S.; Dekio, F.; Wen, J.W. Liver transplantation in a child with multifocal hepatocellular carcinoma hepatitis C and management of post-transplant viral recurrence using boceprevir. Pediatric Transpl. 2014, 18, E64–E68. [Google Scholar] [CrossRef]
- Alsalloom, A. Hepatocellular Carcinoma in a Boy with Progressive familial Intrahepatic Cholestasis Type II: Challenging Identification: Case report. Int. J. Health Sci. 2013, 7, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Seth, S.; Kapoor, A.; Sibal, A. Incidentally detected hepatocellular carcinoma in cirrhotic children. Indian J. Pediatrics 2014, 81, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeop, I.; Taylor, C.J.; Narula, P.; Johnson, L.; Bowen, C.; Gupte, G.L. Hepatocellular carcinoma in a child with intestinal failure-associated liver disease. J. Pediatric Gastroenterol. Nutr. 2012, 54, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; Häberle, B.; Albert, M.H.; Corbacioglu, S.; Fröhlich, B.; Graf, N.; Kammer, B.; Kontny, U.; Leuschner, I.; Scheel-Walter, H.G.; et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatric Blood Cancer 2012, 58, 539–544. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, S.K.; Kwon, C.H.; Joh, J.W.; Choe, Y.H.; Park, C.K. Hepatocellular carcinoma in an infant with biliary atresia younger than 1 year. J. Pediatric Surg. 2012, 47, 819–821. [Google Scholar] [CrossRef]
- Hadžić, N.; Quaglia, A.; Portmann, B.; Paramalingam, S.; Heaton, N.D.; Rela, M.; Mieli-Vergani, G.; Davenport, M. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J. Pediatrics 2011, 159, 617–622.e611. [Google Scholar] [CrossRef]
- Romano, F.; Stroppa, P.; Bravi, M.; Casotti, V.; Lucianetti, A.; Guizetti, M.; Sonzogni, A.; Colledan, M.; D’Antiga, L. Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatric Transpl. 2011, 15, 573–579. [Google Scholar] [CrossRef]
- Masurel-Paulet, A.; Poggi-Bach, J.; Rolland, M.O.; Bernard, O.; Guffon, N.; Dobbelaere, D.; Sarles, J.; Ogier de Baulny, H.; Touati, G. NTBC treatment in tyrosinaemia type I: Long-term outcome in French patients. J. Inherit. Metab. Dis. 2008, 31, 81–87. [Google Scholar] [CrossRef]
- González-Peralta, R.P.; Langham Jr, M.R.; Andres, J.M.; Mohan, P.; Colombani, P.M.; Alford, M.K.; Schwartz, K.B. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J. Pediatric Gastroenterol. Nutr. 2009, 48, 630–635. [Google Scholar] [CrossRef]
- Iida, T.; Zendejas, I.R.; Kayler, L.K.; Magliocca, J.F.; Kim, R.D.; Hemming, A.W.; Gonzalez-Peralta, R.P.; Fujita, S. Hepatocellular carcinoma in a 10-month-old biliary atresia child. Pediatric Transpl. 2009, 13, 1048–1049. [Google Scholar] [CrossRef]
- Riva, S.; Spada, M.; Sciveres, M.; Minervini, M.; Cintorino, D.; Maggiore, G.; Gridelli, B. Hepatocarcinoma in a child with cholesterol ester storage disease. Dig. Liver Dis. 2008, 40, 784. [Google Scholar] [CrossRef] [PubMed]
- Nara, M.; Toyoki, Y.; Hakamada, K.; Narumi, S.; Ishido, K.; Sugai, M.; Munakata, H.; Ito, E.; Sasaki, M. Living donor liver transplantation for a child with recurrent pediatric adult-type hepatocellular carcinoma. Transpl. Proc. 2008, 40, 2828–2829. [Google Scholar] [CrossRef]
- Brunati, A.; Feruzi, Z.; Sokal, E.; Smets, F.; Fervaille, C.; Gosseye, S.; Clapuyt, P.; de Ville de Goyet, J.; Reding, R. Early occurrence of hepatocellular carcinoma in biliary atresia treated by liver transplantation. Pediatric Transpl. 2007, 11, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Morotti, R.A.; Killackey, M.; Shneider, B.L.; Repucci, A.; Emre, S.; Thung, S.N. Hepatocellular carcinoma and congenital absence of the portal vein in a child receiving growth hormone therapy for turner syndrome. Semin. Liver Dis. 2007, 27, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, P.; Fütterer, N.; Lankes, E.; Gempel, K.; Berger, T.M.; Spalinger, J.; Hoerbe, A.; Schwantes, C.; Lindner, M.; Santer, R. Hepatocerebral mitochondrial DNA depletion syndrome caused by deoxyguanosine kinase (DGUOK) mutations. Arch. Neurol. 2006, 63, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Büyükpamukçu, M.; Varan, A.; Haberal, M.; Büyükpamukçu, N.; Köksal, Y.; Coskun, T.; Yüce, A.; Kale, G.; Akyüz, C.; Kutluk, T. The efficacy of liver transplantation in malignant liver tumors associated with tyrosinemia: Clinical and laboratory findings of five cases. Pediatric Transpl. 2006, 10, 517–520. [Google Scholar] [CrossRef]
- Scheers, I.; Bachy, V.; Stephenne, X.; Sokal, E.M. Risk of hepatocellular carcinoma in liver mitochondrial respiratory chain disorders. J. Pediatrics 2005, 146, 414–417. [Google Scholar] [CrossRef]
- Nart, D.; Arikan, C.; Akyildiz, M.; Yuce, G.; Demirpolat, G.; Zeytunlu, M.; Karasu, Z.; Aydoglu, S.; Killi, R.; Yuzer, Y.; et al. Hepatocellular carcinoma in liver transplant era: A clinicopathologic analysis. Transpl. Proc. 2003, 35, 2986–2990. [Google Scholar] [CrossRef]
- Kawasaki, S. Living-donor liver transplantation for hepatocellular carcinoma. Hepato-Gastroenterology 2002, 49, 53–55. [Google Scholar]
- Tatekawa, Y.; Asonuma, K.; Uemoto, S.; Inomata, Y.; Tanaka, K. Liver transplantation for biliary atresia associated with malignant hepatic tumors. J. Pediatric Surg. 2001, 36, 436–439. [Google Scholar] [CrossRef]
- El-Gazzaz, G.; Wong, W.; El-Hadary, M.K.; Gunson, B.K.; Mirza, D.F.; Mayer, A.D.; Buckels, J.A.; McMaster, P. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl. Int. 2000, 13 (Suppl. 1), S406–S409. [Google Scholar] [CrossRef] [PubMed]
- Superina, R.; Bilik, R. Results of liver transplantation in children with unresectable liver tumors. J. Pediatric Surg. 1996, 31, 835–839. [Google Scholar] [CrossRef]
- Ojogho, O.N.; So, S.K.; Keeffe, E.B.; Berquist, W.; Concepcion, W.; Garcia-Kennedy, R.; Imperial, J.; Esquivel, C.O. Orthotopic liver transplantation for hepatocellular carcinoma. Factors affecting long-term patient survival. Arch. Surg. 1996, 131, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Broughan, T.A.; Esquivel, C.O.; Vogt, D.P.; Griffin, G.C.; Norris, D.G. Pretransplant chemotherapy in pediatric hepatocellular carcinoma. J. Pediatric Surg. 1994, 29, 1319–1322. [Google Scholar] [CrossRef]
- Esquivel, C.O.; Gutiérrez, C.; Cox, K.L.; Garcia-Kennedy, R.; Berquist, W.; Concepcion, W. Hepatocellular carcinoma and liver cell dysplasia in children with chronic liver disease. J. Pediatric Surg. 1994, 29, 1465–1469. [Google Scholar] [CrossRef]
- Kawarasaki, H.; Iwanaka, T.; Tsuchida, Y.; Kanamori, Y.; Tanaka, K.; Utsuki, T.; Komuro, H.; Chen, C.L.; Kawasaki, S.; Ishizone, S. Partial liver transplantation from a living donor: Experimental research and clinical experience. J. Pediatric Surg. 1994, 29, 518–522. [Google Scholar] [CrossRef]
- Yandza, T.; Alvarez, F.; Laurent, J.; Gauthier, F.; Dubousset, A.M.; Valayer, J. Pediatric liver transplantation for primary hepatocellular carcinoma associated with hepatitis virus infection. Transpl. Int. 1993, 6, 95–98. [Google Scholar] [CrossRef]
- Salt, A.; Barnes, N.D.; Rolles, K.; Calne, R.Y.; Clayton, P.T.; Leonard, J.V. Liver transplantation in tyrosinaemia type 1: The dilemma of timing the operation. Acta Paediatr. 1992, 81, 449–452. [Google Scholar] [CrossRef]
- Ismail, T.; Angrisani, L.; Gunson, B.K.; Hübscher, S.G.; Buckels, J.A.; Neuberger, J.M.; Elias, E.; McMaster, P. Primary hepatic malignancy: The role of liver transplantation. Br. J. Surg. 1990, 77, 983–987. [Google Scholar] [CrossRef]
- Dehner, L.P.; Snover, D.C.; Sharp, H.L.; Ascher, N.; Nakhleh, R.; Day, D.L. Hereditary tyrosinemia type I (chronic form): Pathologic findings in the liver. Hum. Pathol. 1989, 20, 149–158. [Google Scholar] [CrossRef]
- Finlay, J.; Kalayoglu, M.; Odell, G.; Dinndorf, P.; Frierdich, S. Liver transplantation for primary hepatic cancer in childhood. Lancet 1987, 330, 1086–1087. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Gordon, R.D.; Shaw, B.W., Jr.; Starzl, T.E. Role of liver transplantation in cancer therapy. Ann. Surg. 1985, 202, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otte, J.B.; Meyers, R.L.; de Ville de Goyet, J. Transplantation for liver tumors in children: Time to (re)set the guidelines? Pediatric Transpl. 2013, 17, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, I.A.; Tsoulfas, G. The Evolution of Criteria for Liver Transplantation for Hepatocellular Carcinoma: From Milan to San Francisco and All Around the World! Rev. Fac. Med. Hum. 2017, 17, 56–69. [Google Scholar] [CrossRef]
- Baumann, U.; Adam, R.; Duvoux, C.; Mikolajczyk, R.; Karam, V.; D’Antiga, L.; Chardot, C.; Coker, A.; Colledan, M.; Erizcon, B.G.; et al. Survival of children after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2018, 24, 246–255. [Google Scholar] [CrossRef]
- Ezekian, B.; Mulvihill, M.S.; Schroder, P.M.; Gilmore, B.F.; Leraas, H.J.; Gulack, B.C.; Commander, S.J.; Mavis, A.M.; Kreissman, S.G.; Knechtle, S.J. Improved contemporary outcomes of liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma. Pediatric Transpl. 2018, 22, e13305. [Google Scholar] [CrossRef]
- Mayo, S.C.; Mavros, M.N.; Nathan, H.; Cosgrove, D.; Herman, J.M.; Kamel, I.; Anders, R.A.; Pawlik, T.M. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: A national perspective. J. Am. Coll. Surg. 2014, 218, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161. [Google Scholar] [CrossRef]
- Ninomiya, M.; Shirabe, K.; Facciuto, M.E.; Schwartz, M.E.; Florman, S.S.; Yoshizumi, T.; Harimoto, N.; Ikegami, T.; Uchiyama, H.; Maehara, Y. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J. Am. Coll. Surg. 2015, 220, 297–304. [Google Scholar] [CrossRef]
- Pediatric Hepatic Malignancy International Therapeutic Trial (PHITT). Available online: https://clinicaltrials.gov/ct2/show/NCT03533582 (accessed on 13 March 2021).





| Author | Transplant Center | Country | n |
|---|---|---|---|
| D’Souza, 2020 [22] | Cincinnati Children’s Hospital, Cincinnati | USA | 11 |
| Liu, 2020 [23] | Renji Hospital, Shanghai Jao Tong University, Shanghai | China | 1 |
| Karaca, 2019 [24] | Izmir Kent Hospital, Izmir | Turkey | 6 |
| Waich, 2019 [25] | Medical University of Innsbruck, Innsbruck | Austria | 1 |
| Valamparampil, 2019 [26] | Institute of Liver Disease and Transplantation, Chennai | India | 3 |
| Kumar, 2019 [27] | King Faisal Specialist Hospital, Riyadh | Saudi Arabia | 1 |
| Kang, 2019 [28] | Asan Liver Center, Seoul | South Korea | 1 |
| Timothy, 2019 [29] | Mayo Clinic, Rochester | USA | 1 |
| Tiusanen, 2019 [30] | Helsinki University Hospital, Helsinki | Finland | 5 |
| Cowell, 2019 [31] | Baylor College of Medicine, Houston | USA | 4 |
| Chen, 2018 [32] | Stanford University Medical Center, Palo Alto | USA | 1 |
| Vinayak, 2017 [33] | University of Pittsburgh Medical Center, Pittsburgh | USA | 25 |
| Kohorst, 2017 [34] | Mayo Clinic, Rochester | USA | 2 |
| Khan, 2017 [35] | Washington University, Saint Louis | USA | 2 |
| Geramizadeh, 2017 [36] | Shiraz University of Medical Sciences, Shiraz | Iran | 20 |
| DePasquale, 2017 [37] | Bambino Gesu Pediatric Hospital, Rome | Italy | 3 |
| Troisi, 2017 [38] | Ghent University Medical School, Ghent | Belgium | 1 |
| Haberal, 2017 [39] | Baskent University, Ankara | Turkey | 11 |
| Viswanathan, 2017 [40] | Children’s Hospital at Montefiore, New York | USA | 1 |
| Benedict, 2017 [41] | Yale University School of Medicine, New Haven | USA | 1 |
| Friend, 2017 [42] | University of California, Los Angeles | USA | 2 |
| Imseis, 2017 [43] | University of Texas Health Science Center, Houston | USA | 1 |
| Triana, 2016 [44] | Hospital Universitario La Paz, Madrid | Spain | 4 |
| Shah, 2016 [45] | Bai Jerbai Wadia Hospital, Mumbai | India | 1 |
| Palaniappan, 2016 [46] | Institute of Liver Disease and Transplantation, Chennai | India | 12 |
| Park, 2016 [47] | Seoul National University College of Medicine, Seoul | South Korea | 1 |
| Picoraro, 2016 [48] | Columbia University Medical Center, New York | USA | 1 |
| Pham, 2015 [12] | Stanford University Medical Center, Palo Alto | USA | 10 |
| Abdelfattah, 2015 [49] | King Fasai Specialist Hospital, Riyadh | Saudi Arabia | 4 |
| Yu, 2015 [50] | Seoul National University College of Medicine, Seoul | South Korea | 1 |
| Samuk, 2015 [51] | University of Miami Miller School of Medicine, Miami | USA | 3 |
| Neto, 2014 [52] | Hospital Sirio-Libares, Hospital AC Camargo, Sao Paulo | Brazil | 12 |
| Bartlett, 2014 [53] | Birmingham Children’s Hospital, Birmingham | UK | 1 |
| Malik, 2014 [54] | Children’s Hospital of Philadelphia, Philadelphia | USA | 1 |
| AlSaloom, 2013 [55] | Qassim University, Al-Qassim | Saudi Arabia | 1 |
| Bhatia, 2013 [56] | Indraprastha Apollo Hospital, New Delhi | India | 2 |
| Yeop, 2012 [57] | Birmingham Children’s Hospital, Birmingham | UK | 1 |
| Schmid, 2012 [58] | Multicenter | Germany | 2 |
| Kim, 2012 [59] | Samsung Medical Center, Seoul | South Korea | 1 |
| Hadzic, 2011 [60] | King’s College Hospital, London | UK | 5 |
| Romano, 2011 [61] | San Gerardo Hospital, Milan | Italy | 10 |
| Ismail, 2009 [11] | Children’s Memorial Health Institute, Warsaw | Poland | 9 |
| Masurel Paulet, 2008 [62] | Multicenter | France | 2 |
| Gonzalez-Peralta, 2009 [63] | University of Florida, Gainesville | USA | 1 |
| Iida, 2009 [64] | University of Florida, Gainesville | USA | 1 |
| Riva, 2008 [65] | ISMETT, Palermo | Italy | 1 |
| Nara, 2008 [66] | Hirosaki University School of Medicine, Hirosaki City | Japan | 1 |
| Brunati, 2007 [67] | Saint-Luc University Clinics, Brussels | Belgium | 1 |
| Morotti, 2007 [68] | Mount Sinai School of Medicine, New York | USA | 1 |
| Freisinger, 2006 [69] | Children’s Hospital and Institute of Medical Genetics | Germany | 1 |
| Buyukpamcku, 2006 [70] | Hacettepe Uni Faculty of Medicine, Hacettepe | Turkey | 3 |
| Scheers, 2005 [71] | Saint-Luc University Clinics, Brussels | Belgium | 2 |
| Nart, 2003 [72] | Ege University Medical School, Izmir | Turkey | 6 |
| Kawasaki, 2002 [73] | Shinshu University, Matsumoto | Japan | 3 |
| Tatekawa, 2001 [74] | Kyoto University, Kyoto | Japan | 2 |
| El-Gazzaz, 2000 [75] | Queen Elizabeth Hospital, Birmingham | UK | 2 |
| Superina, 1996 [76] | Hospital for Sick Children, Toronto | Canada | 3 |
| Ojogho, 1996 [77] | Stanford University Medical Center, Palo Alto | USA | 7 |
| Broughan, 1994 [78] | Cleveland Clinic Foundation, Cleveland | USA | 2 |
| Esquivel, 1994 [79] | California Pacific Medical Center, San Francisco | USA | 5 |
| Kawarasaki, 1994 [80] | University of Shinshu Hospital, Matsumoto | Japan | 1 |
| Yandza, 1993 [81] | Hopital Bicetre, Paris | France | 2 |
| Salt, 1992 [82] | Addenbrooke’s Hospital, Cambridge | UK | 2 |
| Ismail, 1990 [83] | Queen Elizabeth Hospital, Birmingham | UK | 1 |
| Dehner, 1989 [84] | University of Minnesota, Minneapolis | USA | 1 |
| Finlay, 1987 [85] | University of Wisconsin, Madison | USA | 1 |
| Iwatsuki, 1985 [86] | University of Colorado, Denver | USA | 7 |
| Variable | Total (n = 245) |
|---|---|
| Clinical Characteristics | |
| Age at liver transplant (years) (n = 185) | 8.1 ± 5.3 |
| Sex (n = 153) | |
| Female | 74 (48.4%) |
| Male | 79 (51.6%) |
| Graft type (n = 137) | |
| Deceased whole | 60 (43.8%) |
| Deceased partial/split | 21 (15.3%) |
| Living | 56 (40.9%) |
| Underlying liver disease overall (n = 226)/in patients with incidental HCC (n = 63) | |
| Tyrosinemia | 77 (34.1%)/26 (41.3%) |
| Biliary Atresia | 25 (11.1%)/9 (14.3%) |
| PFIC | 19 (8.4%)/5 (7.9%) |
| Hepatitis B Virus Infection | 16 (7.0%)/1 (1.6%) |
| Alagille Syndrome | 9 (3.9%)/4 (6.3%) |
| Hepatitis C Virus Infection | 4 (1.8%)/1 (1.6%) |
| Idiopathic Neonatal Hepatitis | 3 (1.3%)/1 (1.6%) |
| A1AT deficiency | 2 (0.9%)/0 (0.0%) |
| Glycogen Storage Disease | 2 (0.9%)/0 (0.0%) |
| Abernethy Syndrome | 2 (0.9%)/2 (3.2%) |
| Meso-caval shunt | 2 (0.9%)/2 (3.2%) |
| DGUOK deficiency | 2 (0.9%)/0 (0.0%) |
| MPV17 deficiency | 2 (0.9%)/0 (0.0%) |
| MRCD | 2 (0.9%)/2 (3.2%) |
| Primary Sclerosing Cholangitis | 1 (0.4%)/1 (1.6%) |
| Autoimmune Hepatitis | 1 (0.4%)/0 (0.0%) |
| Giant Cell Hepatitis | 1 (0.4%)/1 (1.6%) |
| Non-ABC Hepatitis | 1 (0.4%)/1 (1.6%) |
| Wilson Disease | 1 (0.4%)/0 (0.0%) |
| Hemochromatosis | 1 (0.4%)/1 (1.6%) |
| Niemann Pick Disease | 1 (0.4%)/1 (1.6%) |
| Caroli’s Disease | 1 (0.4%)/1 (1.6%) |
| Fibrocystic Disease | 1 (0.4%)/1 (1.6%) |
| Kabuki Syndrome | 1 (0.4%)/0 (0.0%) |
| Turner Syndrome | 1 (0.4%)/1 (1.6%) |
| IFALD | 1 (0.4%)/1 (1.6%) |
| CESD | 1 (0.4%)/0 (0.0%) |
| MDR3 deficiency | 1 (0.4%)/1 (1.6%) |
| NCL | 1 (0.4%)/0 (0.0%) |
| ADA | 1 (0.4%)/0 (0.0%) |
| Cirrhosis (n = 162) | 129 (79.6%) |
| Tumor characteristics | |
| Tumor type (n = 116) | |
| Non-Fibrolamellar | 101 (87.1%) |
| Fibrolamellar | 15 (12.9%) |
| Multiple nodules (n = 198) | 114 (57.6%) |
| Metastasis at Diagnosis (n = 200) | 12 (6.0%) |
| Microvascular Invasion (n = 86) | 42 (48.8%) |
| Macrovascular Invasion (n = 150) | 26 (17.3%) |
| Beyond Milan Criteria (n = 160) | 93 (58.1%) |
| Beyond UCSF Criteria (n = 148) | 70 (47.3%) |
| Pre-LT Treatment | |
| Prior Resection (n = 161) | 15 (0.9%) |
| Prior TACE (n = 148) | 16 (1.0%) |
| Chemotherapy (n = 158) | 50 (31.6%) |
| Cisplatin (n = 142) | 32 (22.5%) |
| Doxorubicin (n = 144) | 28 (19.4%) |
| 5-fluorouracil (n = 142) | 18 (12.7%) |
| Vincristine (n = 144) | 18 (12.5%) |
| Sorafenib (n = 144) | 10 (6.9%) |
| Bevacizumab (n = 144) | 4 (2.8%) |
| Gemcitabine (n = 144) | 3 (2.0%) |
| Oxaliplatin (n = 144) | 3 (2.0%) |
| Irinotecan (n = 144) | 2 (1.3%) |
| Cyclophosphamide (n = 144) | 2 (1.0%) |
| Bleomycin (n = 144) | 2 (1.3%) |
| Post-LT Treatment | |
| Chemotherapy (n = 126) | 30 (23.8%) |
| Doxorubicin (n = 119) | 14 (11.7%) |
| Cisplatin (n = 119) | 13 (10.9%) |
| 5-fluorouracil (n = 117) | 7 (5.9%) |
| Vincristine (n = 119) | 6 (5.0%) |
| Sorafenib (n = 119) | 4 (3.4%) |
| Cyclophosphamide (n = 119) | 3 (2.5%) |
| Bevacizumab (n = 119) | 3 (2.5%) |
| Carboplatin (n = 125) | 3 (2.4%) |
| Nivolumab (n = 119) | 2 (1.6%) |
| Capecitabine (n = 119) | 2 (1.6%) |
| Gemcitabine (n = 119) | 1 (0.8%) |
| Oxaliplatin (n = 119) | 1 (0.8%) |
| Irinotecan (n = 119) | 1 (0.8%) |
| Etoposide (n = 119) | 1 (0.8%) |
| Immunosuppression (n = 67) | |
| Corticosteroids (n = 57) | 56 (98.2%) |
| Tacrolimus (n = 62) | 44 (71.0%) |
| Cyclosporine (n = 59) | 24 (40.7%) |
| Mycophenolate mofetil (n = 62) | 12 (19.4%) |
| Sirolimus (n = 59) | 3 (5.1%) |
| Anti-lymphocyte globulin (n = 59) | 2 (3.4%) |
| Everolimus (n = 59) | 1 (1.7%) |
| Cause of Death | Total (n = 60) |
|---|---|
| Tumor recurrence | 22 (36.7%) |
| Chronic allograft rejection | 5 (8.3%) |
| Sepsis | 5 (8.3%) |
| Primary non-function | 3 (5.0%) |
| Cytomegalovirus infection | 2 (3.3%) |
| Aspiration pneumonia | 1 (1.7%) |
| Respiratory distress and multi-organ failure | 1 (1.7%) |
| Budd-Chiari syndrome | 1 (1.7%) |
| Cardiac arrhythmia | 1 (1.7%) |
| Dialysis-related complication | 1 (1.7%) |
| Hepatic artery thrombosis | 1 (1.7%) |
| Intraoperative cardiac arrest | 1 (1.7%) |
| Metabolic disease | 1 (1.7%) |
| Motor vehicle crash | 1 (1.7%) |
| Post-transplant lymphoproliferative disease | 1 (1.7%) |
| Ruptured pseudoaneurysm | 1 (1.7%) |
| Portal vein thrombosis and intra-operative death during retransplantation | 1 (1.7%) |
| Liver failure (patient also had tumor recurrence) | 1 (1.7%) |
| Unknown | 10 (16.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakos, C.D.; Ziogas, I.A.; Demiri, C.D.; Esagian, S.M.; Economopoulos, K.P.; Moris, D.; Tsoulfas, G.; Alexopoulos, S.P. Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review. Cancers 2022, 14, 1294. https://doi.org/10.3390/cancers14051294
Kakos CD, Ziogas IA, Demiri CD, Esagian SM, Economopoulos KP, Moris D, Tsoulfas G, Alexopoulos SP. Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review. Cancers. 2022; 14(5):1294. https://doi.org/10.3390/cancers14051294
Chicago/Turabian StyleKakos, Christos D., Ioannis A. Ziogas, Charikleia D. Demiri, Stepan M. Esagian, Konstantinos P. Economopoulos, Dimitrios Moris, Georgios Tsoulfas, and Sophoclis P. Alexopoulos. 2022. "Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review" Cancers 14, no. 5: 1294. https://doi.org/10.3390/cancers14051294