1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is projected to be the second cause of cancer death in Western societies within a decade [
1]. The overall 5-year survival is only 6% and in resected patients it is 25%, who only account for 15% of all cases. The extremely poor prognosis of PDAC patients and the aggressive nature of this tumour type lie in its complexity, where genetic and microenvironmental factors interact, determining delayed diagnosis, resistance to chemo-, radio-, and immuno-therapy, and uncontrolled capability to grow and metastasize. Affected by internal or external factors, PDAC cells adopt extensively distinct metabolic processes to meet their demand for growth. Rewired glucose, amino-acid, and lipid metabolism and metabolic crosstalk within the tumour microenvironment contribute to unlimited PDAC progression [
2]. Altered metabolism contributes also to the modulation of apoptosis and autophagy processes and drug targets, conferring a resistant phenotype. In order to overcome resistance to therapies, a variety of experimental compounds inhibiting key metabolic pathways has emerged as a promising approach to potentiate the standard treatments for PDAC in preclinical studies [
3]. These considerations constitute the rational bases to support the concept that the poor prognosis of PDAC patients might be largely improved after employing targeted therapies affecting energy metabolism.
Glycolytic flux is the central carbon metabolism process in all cells, which not only produces adenosine triphosphate (ATP) but also provides metabolic intermediates for anabolic processes that support cell proliferation. Expression levels of glucose transporters and rate-limiting enzymes regulate the rate of glycolytic flux, which is dramatically accelerated in PDAC cells even in the presence of oxygen and normal mitochondrial functionality [
4]. The existence of a link between aerobic glycolysis and tumourigenesis has been known ever since Otto Warburg proposed the “Warburg effect” [
5]. In normal cells, lactate generation through glycolysis is limited to anaerobic conditions, while cancer cells preferentially convert glucose into lactate through glycolysis, even under normal oxygen concentrations [
6]. Lactate production and secretion is coupled with extracellular microenvironment acidification, promoting invasion and metastases and reducing drug efficacy [
7]. In addition, increased glycolytic intermediates can branch out from glycolysis, providing cancer cells with crucial molecules responsible for redox homeostasis, glycosylation, and biosynthesis [
8]. Moreover, the increase in glycolytic flux is a metabolic adaptation of cancer cells to ensure survival and growth in nutrient-deprived environments, which confers to the Warburg effect a key role in the metabolic reprogramming of PDAC cells.
Remarkably, levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were found elevated in human pancreatic adenocarcinomas compared to normal pancreas, as well as in several other cancer tissues [
9]. Furthermore, we previously demonstrated that the oncogenic and mutant isoforms of p53 stabilized GAPDH cytoplasmic localization, preventing its nuclear translocation and, thus, supporting glycolysis of PDAC cells and inhibiting cancer-cell-death mechanisms mediated by nuclear GAPDH [
10]. These and other observations led to an increasing interest in the study and inhibition of GAPDH to achieve a metabolic blockade of cancer cells.
Human GAPDH (
hGAPDH) is a homo-tetramer protein composed of four identical 37-kDa subunits. In the glycolytic process, GAPDH catalyzes the phosphorylation and oxidation of glyceraldehyde-3-phosphate to 1,3-biphosphoglycerate (G-3-P) using NAD
+ as an electron acceptor. Structural studies identified two important functional regions: (i) the glyceraldehyde-3-phosphate catalytic site, including two crucial amino acids involved in the catalytic reaction (Cys
152 and His
179); and (ii) the NAD
+ binding site, a primary structure named the Rossmann fold [
11].
We have previously developed a new class of covalent inhibitors of
Plasmodium falciparum GAPDH (
PfGAPDH), a target for the treatment of malaria, by screening a set of 3-bromo-isoxazoline derivatives that inactivate the enzyme through a covalent, selective bond to the catalytic cysteine [
12,
13]. These 3-Bromo-isoxazoline derivatives were also shown to inhibit human GAPDH, for which they served as tools for the investigation of the conformational flexibility of the enzyme [
14]. In the present study, we tested 3-bromo-isoxazoline derivatives selected from our previous works for antiproliferative effects in PDAC cells, to assess their potential anti-cancer activity.
2. Materials and Methods
2.1. Drugs and Chemicals
All
hGAPDH inhibitors tested (
Table 1) were designed and synthesized in the laboratory of Dr. Paola Conti at the University of Milan, Italy, following procedures previously described [
12,
14]. They were solubilized in absolute methanol or dimethylsulfoxide (DMSO) and stored at −80 °C until use. The autofluorescent dye monodansylcadaverine (MDC) was provided by Sigma-Aldrich (Milan, Italy) and was solubilized in methanol (10 mg/mL); gemcitabine (2′,2′-difluoro-2′-deoxycytidine; GEM) was provided by Accord Healthcare (Milan, Italy) and solubilized in sterile bi-distilled water; annexinV/FITC probe was provided by eBioscience (Thermo Fisher Scientific, Milan, Italy) and solubilized in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, and 2.5 mM CaCl
2).
2.2. Inhibition Assays of Recombinant hGAPDH
Recombinant His-tagged
hGAPDH was produced in Escherichia coli as already described, and covalent inhibition was assayed as reported previously [
14]. Briefly,
hGAPDH at 2 μM concentration was assayed upon incubation for 48 h at 37 °C in a buffered solution containing 10 mM TEA, 5 mM EDTA, and 10 mM sodium arseniate at pH 7.6, in the presence of the inhibitors at 10 µM concentration.
2.3. Cell Cultures
Primary dermal fibroblast from normal human adult (HDFa) and pancreatic adenocarcinoma cell lines (PANC-1 and MIA PaCa-2) were grown in DMEM-Glutamax medium (Thermo Fisher Scientific, Milan, Italy), supplemented with 10% fetal bovine serum (FBS) and 50 µg/mL gentamicin sulfate (all from Gibco, Thermo Fisher Scientific), and incubated at 37 °C with 5% CO2. All cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA).
In order to generate pancreatic-cancer stem cells (CSCs), PDAC parental cell lines (PANC-1 and MIA PaCa-2) were washed twice in PBS (Thermo Fisher Scientific) and then cultured in CSC medium, DMEM/F-12 (US biological Life Sciences, Marblehead, MA, USA), and supplemented with 1 g/L glucose, B27, 1 µg/mL fungizone, 1% penicillin/streptomycin (Thermo Fisher Scientific), 5 µg/mL heparin (Sigma-Aldrich), 20 ng/mL epidermal growth factor (EGF), and 20 ng/mL fibroblast growth factor (FGF) (Peprotech, UK) at 37 °C with 5% CO
2, as previously described by Dalla Pozza et al. [
15].
2.4. Enzyme Assays on Cell Lysates
Cells incubated with the inhibitors at 10 µM concentration were flash-frozen and thawed before the measurements. Cell lysis was produced through three freeze-thaw cycles in 100 µL of a buffered solution containing 200 mM NaCl, 1 mM EDTA, 20 mM CHAPS, and 10% sucrose. The GAPDH activity of cell lysates was measured as reported above and normalized to the total protein content, as measured by the Bradford assay.
2.5. GEPIA Analysis
Gene Expression Profiling Interactive Analysis (GEPIA2,
http://gepia2.cancer-pku.cn (accessed on 22 February 2022) is an open-access online tool for the interactive exploration of RNA sequencing data of 9736 tumours and 8587 normal samples from the TCGA and the Genotype-Tissue Expression (GTEx) programs [
16]. In this study, GEPIA2 was used to calculate the tissue-wise expression of one gene or a multi-gene signature of glycolytic pathway in PDAC, Hexokinase 1 (HK1), Phosphoglucose Isomerase (PHI), Phosphofructokinase 1 (PFK1), Aldolase (ALDOA), Triose Phosphate Isomerase 1 (TPI1), Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Phosphoglycerate Kinase 1 (PGK1), Phosphoglycerate Mutase 1 (PGAM1), Enolase (ENO1), and Pyruvate kinase (PK).
2.6. Cell Proliferation Assay
Cells were seeded in 96-well plate (8 × 103 cells/well for fibroblasts and 5 × 103 cells/well for PDAC cells), and after 24 h they were treated with the various compounds at the concentrations and time points indicated in figure legends. At the end of the treatment period, cell proliferation for parental adherent PDAC cell lines was measured by crystal violet assay in accordance with the protocol of the manufacturer, and absorbance (A595nm) was measured by spectrophotometric analysis (GENios Pro, Tecan, Milan, Italy). Three independent experiments were performed for each assay condition.
Cell proliferation for PDAC CSCs growing in suspension was evaluated using resazurin assay (Immunological Science, Rome, Italy), which is based on the reduction of oxidized non-fluorescent blue resazurin to a red fluorescent dye (resorufin) by the mitochondrial respiratory chain in live cells. Resazurin solution was added in each well, and after 1 h, fluorescence was measured by Tecan GENios Pro Microplate reader (Ex535nm, Em590nm). The quantity of resorufin produced is directly proportional to the amount of living cells. Three independent experiments were performed for each assay condition.
2.7. Western Immunoblotting Analysis
The cells were harvested, washed in PBS, and solubilized in lysis buffer in the presence of phosphatase and protease inhibitors (50 mM Tris–HCl pH 8, 150 mM NaCl, 1% Igepal CA-630, 0.5% Na-Doc, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF, 2.5 mM EDTA, 1 mM PMSF, and 1× protease inhibitor cocktail). After incubation on ice for 30 min, the lysates were centrifuged at 14,000× g for 10 min at 4 °C and the supernatant fractions were used for Western immunoblot analysis. The protein extracts (30 μg/lane) were resolved on a 12% SDS-polyacrylamide gel and electro-blotted onto PVDF membranes (Millipore, Milan, Italy). The membranes were blocked in 5% low-fat milk in TBST (50 mM Tris pH 7.5, 0.9% NaCl, 0.1% Tween 20) for 1 h at room temperature and probed overnight at 4 °C with rabbit monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000) (Cell Signaling, Danvers, MA, USA, #5174S) and mouse monoclonal anti-vinculin (Santa Cruz Biotechnology, Heidelberg, Germany, sc-66305) antibodies. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgGs (1:3000 in blocking solution) (Upstate Biotechnology, Milan, Italy) were used as a secondary antibody. Immunodetection was carried out using chemiluminescent substrates (Amersham Pharmacia Biotech, Milan, Italy) and recorded using a HyperfilmECL (Amersham Pharmacia Biotech).
2.8. L-Lactate Secretion Assay
PANC-1 and MIA PaCa-2 were seeded in 96-well plate (5 × 103 cells/well) and treated with 10 µM of hGAPDH inhibitors (AXP-3009, AXP-3018, AXP-3019) for 48 h. At the end of the treatments, culture medium has been collected, centrifuged at 1500× g for 10 min, and diluted six-fold in distilled H2O. For each sample, 25 μL has been analyzed by L-lactate assay kit (#K-LATE 07/14 Megazyme, Bray, Ireland). The amount of NADH was measured by the increase in absorbance (A340nm). The amount of NADH formed in the reactions is stoichiometric with the amount of L-lactate. L-lactate concentration (g/L) has been calculated in accordance with the instructions of the manufacturer: L-lactate secreted by the cells in each sample was calculated by subtracting the amount of L-lactate in the culture medium (without cells) from the amount of L-lactate in the medium from each sample. The values obtained were normalized to the number of cells in each well, measured by cell-proliferation assay.
2.9. Extracellular Acidification Rate (ECAR) and Oxygen Consumption Rate (OCR) Analysis
ECAR and OCR were measured in PANC-1 and MIA PaCa-2 cell lines by using a Seahorse XFe24 Extracellular Flux Analyzer (Agilent Technologies, Milan, Italy). Cells were seeded at the density of 1.5 × 104 cells/well (PANC-1) or 3.0 × 104 cells/well (MIA PaCa-2) in a V7 XFe24-well cell-culture microplate and treated with 10 µM of AXP-3009, AXP-3018, or AXP-3019 for 48 h, or left untreated. On the day of the assay, cells were incubated in assay medium consisting of Seahorse XF DMEM Medium (Seahorse Bioscience, cat. No. 103575-100) supplemented with 10 mM glucose, 1 mM Sodium Pyruvate, and 2 mM glutamine, pH 7.4, at 37 °C in a non-CO2 incubator for 1 h.
OCR and ECAR were measured at the baseline and after sequentially adding 1 mM oligomycin A (port A), 2 mM (PANC-1) or 1 mM (MIA PaCa-2) of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, port B), and 0.5 mM each of Rotenone and Antimycin A (port C). The non-glycolytic acidification was subtracted from ECAR measurements by adding 50 mM of 2-deoxyglucose (port D). Raw OCR and ECAR data were normalized to the DNA content per well that was quantified with the CyQUANT Cell proliferation assay kit (Thermo Fisher Scientific, cat. No. C35007), in accordance with the instructions of the manufacturer. Data related to mitochondrial respiration were calculated as described [
17]. Data related to glycolysis were calculated as follows: Basal glycolysis: ECAR
BASAL—ECAR
2-DG; glycolytic capacity: ECAR
OLIGO—ECAR
2-DG; glycolytic reserve: ECAR
OLIGO—ECAR
BASAL.
2.10. Apoptotic Assay
PANC-1 and MIA PaCa-2 cell lines were seeded in 96-well plate (5 × 103 cells/well) and the day after were treated with hGAPDH inhibitors as indicated in figure legends and incubated for 48 h. At the end of the treatments, cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, washed twice with PBS, and stained with annexin V/FITC (eBioscience, Thermo Fisher Scientific) diluted in 1X binding buffer [0.1 M Hepes (pH 7.4), 1.4 M NaCl, and 25 mM CaCl2] for 10 min at room temperature in the dark. Cells were then washed with binding buffer, and fluorescence was measured using a multimode plate reader (Ex485nm and Em535nm) (GENios Pro, Tecan). The values were normalized on cell proliferation by crystal violet assay.
2.11. Autophagosome Formation Assay
In order to quantify the autophagic phenomenon, cells were treated with the various compounds and then were incubated with MDC probe, which is a selective dye for acidic vesicular organelles, such as autophagic vacuoles and autophagolysosomes. Briefly, PANC-1 and MIA PaCa-2 cell lines were seeded in 96-well plates (5 × 10
3 cells/well) and treated with the two inhibitors (AXP-3018 and AXP-3019) at 10 μM for 48 h with or without CQ at 1 mM or 3 mM of concentration. At the end of the treatments, cells were incubated in culture medium with 50 μM MDC at 37 °C for 15 min. After incubation, cells were washed with Hanks’ buffer, and fluorescence was measured by using a multimode plate reader (Ex
340nm and Em
535nm) (GENios Pro, Tecan). The values were normalized on cell proliferation by crystal violet assay. Three independent experiments were performed for each assay condition. The study of the autophagic phenomenon has been carried out following the guidelines reported in Klionsky et al. [
18].
2.12. Drug Combination Studies
PANC-1 and MIA PaCa-2 cell lines were seeded in 96-well plate (5 × 10
3 cells/well). After 24 h cells were treated with AXP-3019 and GEM using the molar concentration ratio [AXP-3019]:[GEM] = 1:1. The combination index (CI) was calculated using the Chou-Talalay equation, which takes into account both the potency (IC
50) and the shape of the dose-effect curve by the elaboration of CalcuSyn software (Biosoft, Cambridge, UK), as reported in Fiorini et al. [
19]. The general equation for the classic isobologram is given by CI = (D)1/(Dx)1 + (D)2/(Dx)2 + [(D)1 × (D)2]/[(Dx)1 × (Dx)2], where (Dx)1 and (Dx)2 in the denominators are the doses (or concentrations) of drug 1 and drug 2 alone that give x% growth inhibition, whereas (D)1 and (D)2 in the numerators are the doses of drugs 1 and 2 in combination that also inhibited x% cell-growth inhibition (i.e., isoeffective). CI < 0.3, 0.3 < CI < 0.7, and 0.7 < CI < 1.0 values indicate strong synergism, synergism, or moderate synergism, respectively, whereas, CI = 1 and CI > 1 indicate additive and antagonism, respectively. CI/effect curves represent the CI versus the fraction (0→1) of cells killed by drug combination. The isobologram graphs using IC
25, IC
50, and IC
75 values indicate the equipotent combinations of the two drugs and can be used to further analyze synergism, additivity, or antagonism. Dose-reduction index 50 (DRI
50) represents the dose-reduction folds to obtain 50% cell growth inhibition in a combination setting, as compared to each drug alone. Throughout all experiments, we obtained a linear correlation coefficient of r > 0.90.
2.13. Xenograft Mice Studies
All procedures involving mice were performed in compliance with our institutional animal care guidelines (no. 30/2014-B) and following national and international directives (D.L. 4 March 2014, no. 26; directive 2010/63/EU of the European Parliament and of the Council). MIA PaCa-2 cells (1 × 106 cells/mouse) were subcutaneously injected into the dorsal flank of female nude mice (Charles River Laboratories, Inc., Lecco, Italy). Twelve days after cell inoculation, 5 randomized animals were chosen for each group: control group received 200 μL of PBS vehicle solution, while the treated groups received 20 mg/kg AXP-3009 or AXP-3019 diluted in PBS, by intraperitoneal injection biweekly for 7 weeks. Body mass was recorded weekly for each animal. Tumour size was monitored weekly using a calliper, in two perpendicular dimensions parallel to the surface of the mouse. Tumour volume was calculated using the formula of V = π/6 × [(w × L)^(3/2)]. Animals were sacrificed at the end of the 7-week study period. After euthanizing the mice, the tumours were resected and weighed.
2.14. Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5 software. Significant results were referred with a p value < 0.05. Values are the means of three independent experiments (±SD).
4. Discussion
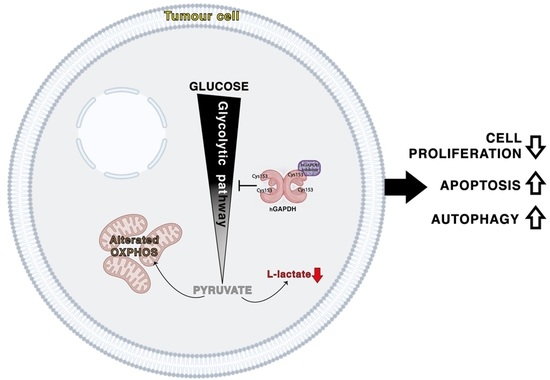
In this study, we demonstrate that the 3-bromo-isoxazoline derivative AXP-3019 is able to inhibit the glycolytic enzyme GAPDH resulting in inhibition of cell proliferation and blockage of tumour-mass growth in a mouse model of human pancreatic adenocarcinoma. Interestingly, whereas several 3-bromo-isoxazoline derivatives structurally related to AXP-3019 behave as effective
hGAPDH inhibitors, when tested against the recombinant isolated enzyme (i.e., AXP-3005, AXP-3009, AXP-3018), their actual ability to inhibit GAPDH intracellularly strongly depends on their chemical structure, which may affect their membrane permeability. Unlike other covalent modifications at the catalytic cysteine, including oxidation and S-nitrosylation [
23], we have previously ruled out that the covalent binding of 3-bromo-isoxazoline derivatives affects the stability and integrity of the GAPDH tetramer [
12]. Therefore, we envisage that the main mechanism of cytotoxicity resides in the inhibition of glycolysis rather than in GAPDH aggregation, a mechanism that was associated with cell death in glioblastoma cells [
24].
In addition, the antiproliferative efficacy of 3-bromo-isoxazoline derivatives is variable depending on the considered cell type, as in the case of AXP-3018, which is inactive in parental cells but more active in CSCs. By further refining the selection of derivatives we have identified two molecules, AXP-3009 and AXP-3019, which are able to efficiently reduce the activity of the recombinant
hGAPDH as well as that of the intracellular enzyme and, consequently, inhibit tumour-cell growth in vitro. Between these two molecules, only compound AXP-3019 was able to produce a significant inhibition of tumour-mass growth in vivo in a xenograft mouse model. This result may be explained by the involvement of detoxification systems, for example at the liver level, that could modify the structure of the AXP-3009 molecule rendering it inactive. We point out further relevant considerations such as the fact that compound AXP-3018, which shows no ability to inhibit the growth of parental PDAC cells, is significantly effective in inhibiting the growth of pancreatic CSCs. CSCs are an unipotent cell population present within the tumour cell mass. CSCs are known to be highly chemoresistant, and in recent years, they have gained intense interest for their tumourigenic and metastatic capacity, playing an integral role in tumour recurrence following chemotherapy [
25]. Consequently, our data on the selective sensitivity of pancreatic CSCs to the AXP-3018 compound appear to be very relevant but need further investigation that may result in the synthesis of additional derivatives capable of making this feature further evident. We hypothesize that this feature is related to the differential composition of the plasma membranes of CSCs compared to parental cells of the same tissue [
21]. An accumulating amount of data has indicated abnormal lipid metabolism in CSCs, and that the alteration of lipid metabolism exerts a great impact on CSCs’ properties such as the capability of self-renewal, differentiation, invasion, metastasis, and drug sensitivity or resistance [
26,
27]. An additional point of reflection that deserves to be discussed is that non-cancerous cells, such as human fibroblasts, are not affected at all by the treatment with any of the tested derivatives, not even with the AXP-3019 that showed the strongest antiproliferative activity in vitro (both in parental PDACs and in CSCs) and murine xenografts. Furthermore, no derivative is able to inhibit the intracellular GAPDH enzyme in fibroblasts. This event might be explained by multiple factors, including the differential composition and characteristic of plasma membranes of non-neoplastic cells, compared to both tumour cells and CSCs. In any case, it is important to note that there is a close correlation between the ability of the various derivatives to inhibit the GAPDH enzyme with that of inhibiting L-lactate secretion and inhibiting cell proliferation. This finding, together with the observation that knockdown of GAPDH expression by siRNA-GAPDH neutralizes the effect of the derivatives, strongly supports the specificity of action of these molecules on the target enzyme. All observations discussed above have been summarized in
Table 3.
The block of glycolytic flux induced by the inhibition of the GAPDH enzyme determines a deep metabolic alteration in the tumour cells. In fact, it is known that cancer cells have a strong metabolic dependence on glycolysis, from which they draw not only most of the ATP produced but also numerous metabolic intermediates for biosynthetic reactions directed to the increase in tumour biomass [
5]. For this purpose, it is also reported that some oncogenes render tumour cells particularly aggressive, by stimulating both glucose uptake and glycolytic flux [
28]. These considerations are further strengthened by our analysis of public databases, showing increased expression of glycolytic enzymes in PDAC patients compared with the normal pancreatic counterpart. Indeed, the rationale of this study is based on this metabolic dependence, showing that PDAC cells blocked in glycolytic flux by 3-bromo-isoxazoline derivatives attempt to compensate for this deficiency with the stimulation of other catabolic pathways, as autophagy flux and OXPHOS. Autophagy is an evolutionarily ancient and highly conserved catabolic process, involving the formation of double-membraned vesicles, called autophagosomes, which engulf cellular proteins and organelles for delivery to the lysosome. Once fused with the lysosome, the contents are degraded, and the macromolecular precursors are recycled or used to fuel metabolic pathways [
29]. Autophagy has opposing, context-dependent roles in cancer and interventions, to both stimulate and inhibit autophagy, which have been proposed as cancer therapies [
30]. In our experimental system, we consider that stimulation of autophagic flux is an adaptation that PDAC cells adopt to compensate for treatment with glycolytic inhibitors. However, under metabolically stressful conditions such as those mentioned, excessive treatment-induced autodigestion itself becomes a cellular stressful event concomitant with the apoptotic process, as demonstrated by the fact that inhibition of autophagy by CQ prevents inhibition of proliferation by 3-bromo-isoxazoline derivatives. Autophagy and apoptosis are likely the main biological phenomena linked with the cell death induced by treatment, as also evidenced by the absence of cytostatic phenomena and alteration of cell cycle phases after treatment of PDAC cells with GAPDH inhibitors (data not shown).
In conclusion, among a panel of differently functionalized 3-bromo-isoxazoline derivatives, we identified AXP-3019 as the best molecule able to inhibit not only recombinant hGAPDH but also the intracellular enzyme in PDAC cells and related CSCs, without affecting GAPDH enzymatic activity in fibroblasts and their proliferation. Furthermore, AXP-3019 strongly neutralizes tumour-mass growth without apparent toxic effects in mice. Among the biological phenomena associated with the treatment, we noted the reduction in L-lactate secretion, stimulation of apoptosis, and autophagy. Finally, we demonstrated a strong antiproliferative synergy between AXP-3019 and the standard drug gemcitabine, especially at low concentrations, suggesting a possible reduction in chemotherapy doses in PDAC patients with a decrease in side effects. Finally, these data further support the connection between chemoresistance and the metabolic reprogramming of tumours, a concept that is accumulating an ever-increasing amount of scientific evidence.

































 inhibition; = no significance difference.
inhibition; = no significance difference.