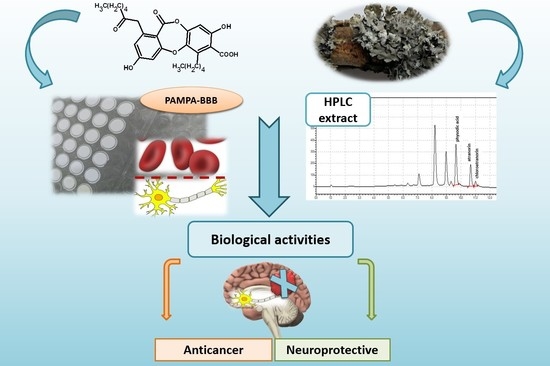
Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Solvents
2.3. Preparation of Extract
2.4. Determination of Cytotoxicity of Physodic Acid and H. Physodes Acetone Extract
2.5. Anti-Hyaluronidase Activity
2.6. Anticyclooxygenase Activity
2.7. Anti-Tyrosinase Activity
2.8. Anticholinesterase Activity
2.9. Antioxidant Activity
2.10. Total Phenolic Content (TPC)
2.11. High-Performance Liquid Chromatography (HPLC) Analysis
2.12. Permeability through the Blood–Brain-Barrier (PAMPA-BBB)
2.13. Statistical Analysis
3. Results
3.1. Screening of Biological Activity
3.1.1. Cytotoxic Activity Against Glioblastoma Cells
3.1.2. Anti-Hyaluronidase Activity
3.1.3. Inhibition of COX-2
3.1.4. Anti-Tyrosinase Activity
3.1.5. Anticholinesterase Activity
3.1.6. Antioxidant Activity
3.2. Phytochemical Analysis
3.2.1. Total Polyphenols Content (TPC)
3.2.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.3. Permeability through the Blood–Brain-Barrier (PAMPA-BBB)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majchrzak-Celinska, A.; Zielinska-Przyjemska, M.; Wierzchowski, M.; Kleszcz, R.; Studzinska-Sroka, E.; Kaczmarek, M.; Paluszczak, J.; Cielecka-Piontek, J.; Krajka-Kuzniak, V. Methoxy-stilbenes downregulate the transcription of Wnt/β-catenin-dependent genes and lead to cell cycle arrest and apoptosis in human T98G glioblastoma cells. Adv. Med. Sci. 2020, 66, 6–20, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Goozee, K.; Shah, T.; Sohrabi, H.; Rainey-Smith, S.; Brown, B.; Verdile, G.; Martins, R. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016, 115, 449–465. [Google Scholar] [CrossRef]
- Moody, R.; Wilson, K.; Jaworowski, A.; Plebanski, M. Natural compounds with potential to modulate cancer therapies and self-reactive immune cells. Cancers 2020, 12, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancato, V.; Nuzzo, S.; Tramontano, L.; Condorelli, G.; Salvatore, M.; Cavaliere, C. Predicting survival in glioblastoma patients using diffusion MR imaging metrics—A systematic review. Cancers 2020, 12, 2858. [Google Scholar] [CrossRef]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural products in neurodegenerative diseases: A great promise but an ethical challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila, M. Neuromelanin, aging, and neuronal vulnerability in Parkinson’s disease. Mov. Disord. 2019, 34, 1440–1451. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chen, C.-M. The role of oxidative stress in Parkinson’s disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015, 16, 193–217. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.E.; Pedron, S.; Shyu, P.; Hu, Y.; Sarkaria, J.N.; Harley, B.A.C. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front. Mater. 2018, 5, 39. [Google Scholar] [CrossRef]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm Sci. 2013, 8, 233–242. [Google Scholar] [PubMed]
- Yu, H.; Wang, Q.; Sun, Y.; Shen, M.; Li, H.; Duan, Y. A new PAMPA model proposed on the basis of a synthetic phospholipid membrane. PLoS ONE 2015, 10, e0116502. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, Q.; Tian, Y.; Xiao, S.; Jin, T.; Fan, X. A metabonomic characterization of (+)-usnic acid-induced liver injury by gas chromatography—Mass spectrometry-based metabolic orofiling of the plasma and liver in rat. Int. J. Toxicol. 2011, 30, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.K.; Rath, O.; Clayton, E.; Tomasi, S.; Kozielski, F. Depsidones from lichens as natural product inhibitors of M-phase phosphoprotein 1, a human kinesin required for cytokinesis. J. Nat. Prod. 2016, 79, 1576–1585. [Google Scholar] [CrossRef]
- Studzinska-Sroka, E.; Dubino, A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018, 64, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Rankovic, B.; Kosanic, M. Lichens as a potential source of bioactive secondary metabolites. In Lichen Secondary Metabolites; Rankovic, B., Ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Solarova, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Busselberg, D.; Solar, P. Anticancer potential of lichens’ secondary metabolites. Biomolecules 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Moriano, C.; Divakar, P.K.; Crespo, A.; Gomez-Serranillos, M.P. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Togar, B.; Turkez, H. In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm. Biol. 2016, 54, 1748–1762. [Google Scholar] [CrossRef] [Green Version]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Emsen, B.; Sadi, G.; Bostanci, A.; Aslan, A. In vitro evaluation of cytotoxic, oxidative, genotoxic, and apoptotic activities of physodic acid from Pseudevernia furfuracea in HepG2 and THLE2 cells. Plant Biosyst. 2020, 1–10. [Google Scholar] [CrossRef]
- Studzinska-Sroka, E.; Zarabska-Bozejewicz, D. Hypogymnia physode—A lichen with interesting medicinal potential and ecological properties. J. Herb. Med. 2019, 17–18, 100287. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kleszcz, R.; Studzinska-Sroka, E.; Krajka-Kuzniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.; Pongor, L.; Su, Y.T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a clinical biomarker in glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Podolak, I.; Galanty, A.; Zaluski, D.; Makowska-Was, J.; Sobolewska, D.; Janeczko, Z.; Zmudzki, P. In vitro anti-denaturation and anti-hyaluronidase activities of extracts and galactolipids from leaves of Impatiens parviflora DC. Nat. Prod. Res. 2016, 30, 1219–1223. [Google Scholar] [CrossRef]
- Lim, T.Y.; Lim, Y.Y.; Yule, C.M. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]
- Ellman, G.L.; Lourtney, D.K.; Andres, V.; Gmelin, G. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wojcik, E. Terpenes and phenylpropanoids as acetyl- and butyrylcholinesterase inhibitors: A comparative study. Curr Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Wlodarczyk, A.; Studzinska-Sroka, E.; Zuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studzinska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Luczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-inflammatory activity and phytochemical profile of Galinsoga Parviflora cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.L.; Balimane, P.V. A novel design of artificial membrane for improving the PAMPA model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Latacz, G.; Lubelska, A.; Jastrzebska-Wiesek, M.; Partyka, A.; Marc, M.A.; Satala, G.; Wilczynska, D.; Kotanska, M.; Wiecek, M.; Kaminska, K.; et al. The 1,3,5-triazine derivatives as innovative chemical family of 5-HT6 serotonin receptor agents with therapeutic perspectives for cognitive impairment. Int. J. Mol. Sci. 2019, 20, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialonska, D.; Dayan, F.E. Chemistry of the lichen Hypogymnia physodes transplanted to an industrial region. J. Chem Ecol. 2005, 31, 2975–2991. [Google Scholar] [CrossRef]
- Rankovic, B.; Kosanic, M.; Manojlovic, N.; Rancic, A.; Stanojkovic, T. Chemical composition of Hypogymnia physodes lichen and biological activities of some its major metabolites. Med. Chem. Res. 2014, 23, 408–416. [Google Scholar] [CrossRef]
- Latkowska, E.; Bober, B.; Chrapusta, E.; Adamski, M.; Kaminski, A.; Bialczyk, J. Secondary metabolites of the lichen Hypogymnia physodes (L.) Nyl. and their presence in spruce (Picea abies (L.) H. Karst.) bark. Phytochemistry 2015, 118, 116–123. [Google Scholar] [CrossRef]
- Olivier-Jimenez, D.; Chollet-Krugler, M.; Rondeau, D.; Beniddir, M.A.; Ferron, S.; Delhaye, T.; Allard, P.M.; Wolfender, J.L.; Sipman, H.J.M.; Lücking, R.; et al. A database of high-resolution MS/MS spectra for lichen metabolites. Sci. Data 2019, 6, 294. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Moriano, C.; Gomez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Piotrowska, H.; Kucinska, M.; Murias, M.; Bylka, W. Cytotoxic activity of physodic acid and acetone extract from Hypogymnia physodes against breast cancer cell lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.; Costa, A.; Osorio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current standards of care in glioblastoma therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; pp. 197–241. Available online: https://www.ncbi.nlm.nih.gov/books/NBK469987/ (accessed on 10 February 2021).
- Yoshino, A.; Ogino, A.; Yachi, K.; Ohta, T.; Fukushima, T.; Watanabe, T.; Katayama, Y.; Okamoto, Y.; Naruse, N.; Sano, E.; et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int. J. Oncol. 2010, 36, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Devehat, F.L.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural compounds for the prevention and treatment of cardiovascular and neurodegenerative diseases. Foods 2020, 10, 29. [Google Scholar] [CrossRef]
- Jin, S.G.; Jeong, Y.I.; Jung, S.; Ryu, H.H.; Jin, Y.H.; Kim, I.Y. The effect of hyaluronic acid on the invasiveness of malignant glioma cells: Comparison of invasion potential at hyaluronic acid hydrogel and matrigel. J. Korean Neurosurg. Soc. 2009, 46, 472–478. [Google Scholar] [CrossRef]
- Bruno, M.; Trucchi, B.; Burlando, B.; Ranzato, E.; Martinotti, S.; Akkol, E.K.; Suntar, I.; Keles, H.; Verotta, L. (+)-Usnic acid enamines with remarkable cicatrizing properties. Bioorg. Med. Chem. 2013, 21, 1834–1843. [Google Scholar] [CrossRef]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. ChemMedChem 2012, 7, 2077–2081. [Google Scholar] [CrossRef]
- Jiang, J.; Qiu, J.; Li, Q.; Shi, Z. Prostaglandin E2 signaling: Alternative target for glioblastoma? Trends Cancer 2017, 3, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Bartels, A.L.; Leenders, K.L. Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr. Neuropharmacol. 2010, 8, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, M.; Miura, Y.; Boohene, J.; Kinoshita, Y.; Yamamoto, Y.; Yoshimura, I.; Yamada, Y. Inhibition of tyrosine activity by cultured lichen tissues and bionts. Planta Med. 1993, 59, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, G.; Zlatanovic, I.; Zrnzevic, I.; Stankovic, M.; Stankov Jovanovic, V.; Zlatkovic, B. Hypogymnia tubulosa extracts: Chemical profile and biological activities. Nat. Prod. Res. 2018, 32, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Hawryl, A.; Hawryl, M.; Hajnos-Stolarz, A.; Abramek, J.; Bogucka-Kocka, A.; Komsta, L. HPLC Fingerprint Analysis with the antioxidant and cytotoxic activities of selected lichens combined with the chemometric calculations. Molecules 2020, 25, 4301. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Lind, M.; Nybakken, L.; Gauslaa, Y. Possible functional roles of cortical depsides and medullary depsidones in the foliose lichen Hypogymnia physodes. Flora 2009, 204, 40–48. [Google Scholar] [CrossRef]
- Naoi, M.; Shamoto-Nagai, M.; Maruyama, W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019, 14, FNL9. [Google Scholar] [CrossRef] [Green Version]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011, 68, 1385–1392. [Google Scholar] [CrossRef] [Green Version]








| Type of Cells | H. Physodes Extract | Physodic Acid |
|---|---|---|
| IC50 [µg/mL] | IC50 [µg/mL (µM)] | |
| A-172 | 61.37 ± 5.19 | 19.95 ± 0.59 (42.41 ± 1.25) |
| T98G | 72.15 ± 4.33 | 23.79 ± 0.51 (50.57 ± 1.09) |
| U-138MG | 68.36 ± 1.58 | 21.51 ± 1.98 (45.72 ± 4.20) |
| Concentration (Final Concentration) | Inhibition of COX-2 | ||
|---|---|---|---|
| H. physodes extract | Physodic acid | H. physodes extract | Physodic acid |
| 152 µg/mL | 100 µg/mL | n.a. | n.a. |
| 304 µg/mL | 200 µg/mL | 28.60% ± 2.4% | n.a. |
| 456 µg/mL | 300 µg/mL | 52.40% ± 1.1% | n.a. |
| Materials for Studies | Inhibition of Enzyme | |
|---|---|---|
| AChE | BChE | |
| H. physodes extract 72.5 µg/mL | 9.6 ± 0.1% | n.a. |
| Physodic acid 72.5 µg/mL (154 µM) | n.a. | 8.1 ± 0.2% |
| Inhibition similar to: | eserine at 0.12 ± 0.007 μg/mL | eserine at 0.019 ± 0.001 μg/mL |
| donepezil at 0.30 ± 0.013 μg/mL | donepezil at 0.026 ± 0.014 μg/mL | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. https://doi.org/10.3390/cancers13071717
Studzińska-Sroka E, Majchrzak-Celińska A, Zalewski P, Szwajgier D, Baranowska-Wójcik E, Żarowski M, Plech T, Cielecka-Piontek J. Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers. 2021; 13(7):1717. https://doi.org/10.3390/cancers13071717
Chicago/Turabian StyleStudzińska-Sroka, Elżbieta, Aleksandra Majchrzak-Celińska, Przemysław Zalewski, Dominik Szwajgier, Ewa Baranowska-Wójcik, Marcin Żarowski, Tomasz Plech, and Judyta Cielecka-Piontek. 2021. "Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System" Cancers 13, no. 7: 1717. https://doi.org/10.3390/cancers13071717