Role and Merits of Green Based Nanocarriers in Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
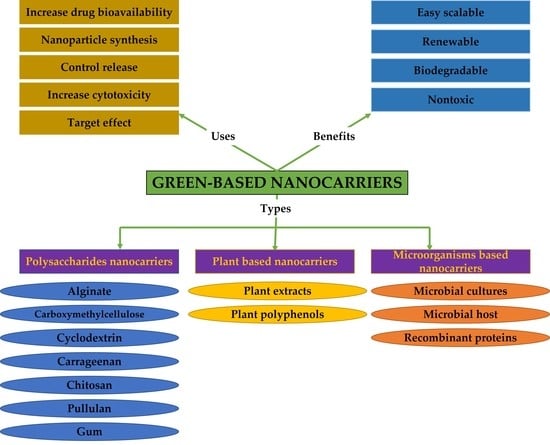
2. Green Components in Nanocarriers for Anticancer Therapy
2.1. Polysaccharides-Based Nano Delivery Systems
2.2. Plant Extracts-Based Nanocarriers
2.3. Plant Polyphenols and Pospholipids-Based Nanocarriers
2.4. Microorganisms-Based Nanocarriers
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374. [Google Scholar] [CrossRef]
- Raliya, R.; Chadha, T.S.; Hadad, K.; Biswas, P. Perspective on nanoparticle technology for biomedical use. Curr. Pharm. Des. 2016, 22, 2481–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Future Med. 2012, 7, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.C. Size and Shape Dependent Second Order Nonlinear Optical Properties of Nanomaterials and Their Application in Biological and Chemical Sensing. Chem. Rev. 2010, 110, 5332–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Garcia, E.; Sarkar, A.; Kapoor, S.; Rafiq, K.; Chand, H.; Jayant, R. Nanoparticle Based Treatment for Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A. The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol. Rev. 2016, 5, 601–622. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; He, B.; Zhao, L. Fabrication of hydrophobic biocomposite by combining cellulosic fibers with polyhydroxyalkanoate. Cellulose 2017, 24, 2265–2274. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Chaabouni, E.; Gassara, F.; Brar, S.K. Biopolymers Synthesis and Application. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 415–443. [Google Scholar]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-Based Dosage Forms: The Next Generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Zhao, X.; Tang, W.; Wang, F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci. Rep. 2019, 9, 6754. [Google Scholar] [CrossRef] [PubMed]
- Pavli, M.; Baumgartner, S.; Kos, P.; Kogej, K. Doxazosin-carrageenan interactions: A novel approach for studying drug-polymer interactions and relation to controlled drug release. Int. J. Pharm. 2011, 421, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.; Mosallanezhad, A.; Soleymani, M.; Sabzi, M. Magnetic- and pH-responsive κ-carrageenan/chitosan complexes for controlled release of methotrexate anticancer drug. Int. J. Biol. Macromol. 2017, 97, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Atlasi, Z.; Mahdavinia, G.R.; Hadifar, S.; Sabzi, M. Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release. Mater. Sci. Eng. C 2021, 124, 112042. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.; Lee, K.X.; Teow, S.-Y. Green Synthesized Montmorillonite/Carrageenan/Fe3O4 Nanocomposites for pH-Responsive Release of Protocatechuic Acid and Its Anticancer Activity. Int. J. Mol. Sci. 2020, 21, 4851. [Google Scholar] [CrossRef]
- Donadelli, M.; Costanzo, C.; Beghelli, S.; Scupoli, M.; Dandrea, M.; Bonora, A.; Piacentini, P.; Budillon, A.; Caraglia, M.; Scarpa, A.; et al. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim. Biophys. Acta 2007, 1773, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Godugu, D.; Beedu, S. Synthesis, characterisation and anti-tumour activity of biopolymer based platinum nanoparticles and 5-fluorouracil loaded platinum nanoparticles. IET Nanobiotechnol. 2019, 13, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Sericin/RBA embedded gellan gum based smart nanosystem for pH responsive drug delivery. Int. J. Biol. Macromol. 2018, 120, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Reddy, E.; Shiras, A.; Pokharkar, V.; Prasad, B. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry 2008, 14, 10244–10250. [Google Scholar] [CrossRef]
- Barthold, S.; Hittinger, M.; Primavessy, D.; Zapp, A.; Groß, H.; Schneider, M. Preparation of maltodextrin nanoparticles and encapsulation of bovine serum albumin-Influence of formulation parameters. Eur. J. Pharm. Biopharm. 2019, 142, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Generalov, R.; Pereira Mdo, C.; Peres, I.; Juzenas, P.; Coelho, M. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Rathee, P.; Sharma, K.; Chaugule, B.B.; Kar, N.; Bera, T. Immuno-modulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013, 57, 50–56. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef] [PubMed]
- Venkatpurwar, V.; Shiras, A.; Pokharkar, V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm. 2011, 409, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-M.; Cao, Y.; Yang, Y.; Chen, J.-T.; Liu, Y. A small-sized graphene oxide supramolecular assembly for targeted delivery of camptothecin. Chem. Commun. 2014, 50, 13066–13069. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, J.; Zhang, H.; Qin, Y.; Xu, X.; Jin, Z. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal-Organic Frameworks Based on Short-Chain Starch Nanoparticles. J. Agric. Food Chem. 2018, 66, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Deng, Y.; Zhao, Z.; Li, X.; Xia, Y. Facile Synthesis of Gold Nanoparticles with Alginate and Its Catalytic Activity for Reduction of 4-Nitrophenol and H2O2 Detection. Materials 2017, 10, 557. [Google Scholar] [CrossRef]
- Dey, S.; Sherly, M.C.D.; Rekha, M.R.; Sreenivasan, K. Alginate stabilized gold nanoparticle as multidrug carrier: Evaluation of cellular interactions and hemolytic potential. Carbohydr. Polym. 2016, 136, 71–80. [Google Scholar] [CrossRef]
- Catley, B.J.; Ramsay, A.; Servis, C. Observations on the structure of the fungal extracellular polysaccharide, pullulan. Carbohydr. Res. 1986, 153, 79–86. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Malhotra, A.; Bhattacharjee, P.; Prasad, G.S. Facile and rapid thermo-regulated biomineralization of gold by pullulan and study of its thermodynamic parameters. Carbohydr. Polym. 2014, 106, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Laksee, S.; Puthong, S.; Teerawatananond, T.; Palaga, T.; Muangsin, N. Highly efficient and facile fabrication of monodispersed Au nanoparticles using pullulan and their application as anticancer drug carriers. Carbohydr. Polym. 2017, 173, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Laksee, S.; Sansanaphongpricha, K.; Puthong, S.; Sangphech, N.; Palaga, T.; Muangsin, N. New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int. J. Biol. Macromol. 2020, 162, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, C.; Omer, A.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Behzadi Nia, S.; Namazi, H. Green encapsulation of LDH(Zn/Al)-5-Fu with carboxymethyl cellulose biopolymer; new nanovehicle for oral colorectal cancer treatment. Int. J. Biol. Macromol. 2019, 139, 994–1001. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Amaral-Júnior, J.C.; Carvalho, S.M.; Carvalho, I.C.; Mansur, H.S. Cu-In-S/ZnS@carboxymethylcellulose supramolecular structures: Fluorescent nanoarchitectures for targeted-theranostics of cancer cells. Carbohydr. Polym. 2020, 247, 116703. [Google Scholar] [CrossRef] [PubMed]
- Samarehfekri, H.; Rahimi, H.R.; Ranjbar, M. Controlled and cellulose eco-friendly synthesis and characterization of Bi2O2CO3 quantum dot nanostructures (QDNSs) and drug delivery study. Sci. Rep. 2020, 10, 21302. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Lather, V.; Pandita, D. A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery. Int. J. Biol. Macromol. 2016, 84, 380–384. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C. Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dinesh, M.G.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B Biointerfaces 2013, 106, 208–216. [Google Scholar] [CrossRef]
- Chinnaiyan, S.; Soloman, A.; Perumal, R.; Gopinath, A.; Balaraman, M. 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol. 2019, 13, 824–828. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Patil, R.V.; Havaldar, D.V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnol. 2021, 19, 84. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Mohamad, S.E.; Yew, Y.P.; Isa, E.D.M.; Yap, H.-Y.; Lim, W.L.; Teow, S.-Y. Bio-Mediated Synthesis and Characterisation of Silver Nanocarrier, and Its Potent Anticancer Action. Nanomaterials 2019, 9, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Owens, G.; Chen, Z. Modified green synthesis of Fe3O4@SiO2 nanoparticles for pH responsive drug release. Mater. Sci. Eng. C 2020, 112, 110900. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Mahjoub, S.; Elahi, S.; Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf. B Biointerfaces 2020, 186, 110686. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xu, Z.; Wang, Y.; Ke, Y.; Jiang, Z.; Yuan, Z.; Li, H. Role of polyphenols in plant-mediated synthesis of gold nanoparticles: Identification of active components and their functional mechanism. Nanotechnology 2020, 31, 415601. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Megharaj, M. Characterization of Iron–Polyphenol Nanoparticles Synthesized by Three Plant Extracts and Their Fenton Oxidation of Azo Dye. ACS Sustain. Chem. Eng. 2014, 2, 1022–1025. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Liao, X.; Shi, B. One-step, size-controlled synthesis of gold nanoparticles at room temperature using plant tannin. Green Chem. 2010, 12, 395–399. [Google Scholar] [CrossRef]
- Mao, H.; Liao, Y.; Ma, J.; Zhao, S.L.; Huo, F.W. Water-soluble metal nanoparticles stabilized by plant polyphenols for improving the catalytic properties in oxidation of alcohols. Nanoscale 2015, 8, 1049–1054. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Shang, L.; Yang, C.; Kong, L.; Zhang, Z. Green tea extract-assembled nanoclusters for combinational photothermal and chemotherapy. J. Mater. Chem. B 2019, 7, 5972–5982. [Google Scholar] [CrossRef]
- Wang, X.; Hao, L.; Zhang, C.; Chen, J.; Zhang, P. High efficient anti-cancer drug delivery systems using tea polyphenols reduced and functionalized graphene oxide. J. Biomater. Appl. 2017, 31, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Kumar, V.; Caligiuri, I.; Ambrosi, E.; Pontoglio, E.; Agostini, M.; Tuccinardi, T.; Benedetti, A.; et al. Bottom-up synthesis of carbon nanoparticles with higher doxorubicin efficacy. J. Control. Release 2017, 248, 144–152. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.; Nadeem, M.; Peters, D.; Mubarak, M. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Güder, A.; Korkmaz, H.; Gökce, H.; Alpaslan, Y.B.; Alpaslan, G. Isolation, characterization, spectroscopic properties and quantum chemical computations of an important phytoalexin resveratrol as antioxidant component from Vitis labrusca L. and their chemical compositions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Tomoaia, G.; Horovitz, O.; Mocanu, A.; Nita, A.; Avram, A.; Racz, C.P.; Soritau, O.; Cenariu, M.; Tomoaia-Cotisel, M. Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf. B Biointerfaces 2015, 135, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Ghosh, A.; Ghosh, C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef]
- Liang, K.; Ng, S.; Lee, F.; Lim, J.; Chung, J.; Lee, S.; Kurisawa, M. Targeted intracellular protein delivery based on hyaluronic acid-green tea catechin nanogels. Acta Biomater. 2016, 33, 142–152. [Google Scholar] [CrossRef]
- Bae, K.; Tan, S.; Yamashita, A.; Ang, W.; Gao, S.; Wang, S.; Chung, J.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, X.; Tian, X.; Zhang, P.; Chen, Z.; Hu, X.; Mei, X. GSH and enzyme responsive nanospheres based on self-assembly of green tea polyphenols and BSA used for target cancer chemotherapy. Colloids Surf. B. Biointerfaces 2019, 173, 654–661. [Google Scholar] [CrossRef]
- Soreide, K.; Janssen, E.; Körner, H.; Baak, J. Trypsin in colorectal cancer: Molecular biological mechanisms of proliferation, invasion, and metastasis. J. Pathol. 2006, 209, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C. Mixed-chain phospholipids: Structures and chain-melting behavior. Lipids 2001, 36, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneim, H.A.; Xiaoqiang, Z.; Sherif, M.A.; Sameh, A.K.; Qingzhe, J.; Xingguo, W. Natural phospholipids: Occurrence, biosynthesis, separation, identification, and beneficial health aspects. Crit. Rev. Food Sci. Nutr. 2017, 59, 253–275. [Google Scholar] [CrossRef]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (-)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; He, W.; Wang, X.-Q.; Hu, X.-M. Preparation and efficacy of tumor vasculature-targeted doxorubicin cationic liposomes coated by N-trimethyl chitosan. J. Appl. Polym. Sci. 2011, 121, 2149–2156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Zhang, H.; Han, J.; Zhang, L.; Lin, Q.; Ai, F. Carboxymethyl chitosan/phospholipid bilayer-capped mesoporous carbon nanoparticles with pH-responsive and prolonged release properties for oral delivery of the antitumor drug, Docetaxel. Int. J. Pharm. 2017, 532, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Pierigè, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef]
- Luo, C.; Huang, C.; Su, C.; Yeh, C. Bacteria-Mediated Hypoxia-Specific Delivery of Nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016, 16, 3493–3499. [Google Scholar] [CrossRef]
- Ma, X.; Liu, P.; Tian, Y.; Zhu, G.; Yang, P.; Wang, G.; Yang, L. A mineralized cell-based functional platform: Construction of yeast cells with biogenetic intracellular hydroxyapatite nanoscaffolds. Nanoscale 2018, 10, 3489–3496. [Google Scholar] [CrossRef]
- Seo, J.M.; Kim, E.B.; Hyun, M.S.; Kim, B.B.; Park, T.J. Self-assembly of biogenic gold nanoparticles and their use to enhance drug delivery into cells. Colloids Surf. B Biointerfaces 2015, 135, 27–34. [Google Scholar] [CrossRef]
- Ganesh Kumar, C.; Poornachandra, Y.; Mamidyala, S.K. Green synthesis of bacterial gold nanoparticles conjugated to resveratrol as delivery vehicles. Colloids Surf. B Biointerfaces 2014, 123, 311–317. [Google Scholar] [CrossRef]






| Examples of Green Component in Nanocarriers | Function |
|---|---|
| Alginate |
|
| CMC |
|
| CD |
|
| Chitosan |
|
| CR |
|
| Gum |
|
| HA |
|
| MMt |
|
| Phospholipids |
|
| Plant extracts |
|
| Pullulan |
|
| Formulation | Size (nm) * | Shape of the NPs/Formulation | Composition ** | Drug Loaded | Advantage of Using the Nanocarrier | References |
|---|---|---|---|---|---|---|
| Magnetic nanocomposite | 141 ± 6 | Spherical and ellipsoidal shape | CR oligosaccharides/GNPs | Epirubicin | Increased cytotoxicity | [16] |
| 4 for the Fe3O4 NPs | Spherical Fe3O4 NPs | Fe3O4 NPs/CR/chitosan | Methotrexate | Controlled release | [18] | |
| 9.2 ± 1.3 for Fe3O4 NPs (TEM) | Sheet-like matrix with spherical Fe3O4 NPs | CR/MMt/F3O4 NPs | Protocatechuic acid | Targeted release | [20] | |
| Not reported | Irregular and coarse nanogel with spherical Fe3O4 NPs | CR/m-MMt/chitosan/Fe3O4 NPs | Sunitinib | Controlled release | [19] | |
| <50 (TEM) | Dispersed | Fe3O4 NPs/E. cochinchensis leaf extract/mesoporous silica surface modification with carboxyl groups | DOX | Controlled and targeted release | [58] | |
| Nanocomposite | 6.4 nm in height (Atomic Force Microscopy (AFM)) | Nanosheet | CD/graphene oxide sheets/hyaluronated adamantane | Camptothecin | Increased cytotoxicity and targeted release | [32] |
| 171.25 nm in height (AFM) | Nanosheet | Tea polyphenols/graphene oxide sheets | DOX | Increased cytotoxicity and target release | [66] | |
| 17 | Spherical | Black tea aqueous extract/CNPs | DOX | Increased cytotoxicity and target release | [67] | |
| 202 | Spherical | EGCG/BSA/FA | DOX | Increased cytotoxicity and target release | [75] | |
| 225 | Spherical | Sericin/rice bran albumin/gellan gum | DOX | Sustained and controlled release | [24] | |
| Variable sizes (70 ± 30 to 143 ± 36) depending on the plant extract used | Spherical | S. cumini,B. variegata, C. deodara, L. japonica and E. sphaericus/poly(D,L-lactide) NPs | Quercetin | Controlled release | [48] | |
| 375 | Spherical | Acrysol oil/PLGA NPs | resveratrol | Increased cytotoxicity and sustained release | [49] | |
| 120 ± 28 (DLS) | Spherical | Maltodextrin/gum arabic | EGCG | Increased cytotoxicity | [27] | |
| 187 (DLS) | Well dispersed | Alginate-curcumin/GNPs | Methotrexate and curcumin | Increased cytotoxicity | [36] | |
| 80 nm in height (AFM) | Spongy structure withsquare and lamellar shapes on the graphene sheets | CMC-Zinc metal framework/graphene oxide sheets | DOX | Controlled and sustained release | [43] | |
| 4–5 mm for wet beads (visual) | Spherical | CMC/chitosan/ZnONPs | 5-FU | Controlled release | [44] | |
| 7–9 mm for wet beads (SEM) | Rough surface | CMC/layered double hydroxide (zinc/aluminum) | 5-FU | Controlled release | [45] | |
| Variable according to type of chitosan used | Spherical | Lecithin/N-trimethyl chitosan | DOX | Increased cytotoxicity and targeting release | [82] | |
| MNPs | 20–25 (TEM) | Spherical | Gum karaya/GNPs | gemcitabine hydrochloride | Increased cytotoxicity | [22] |
| 13 ± 1 (TEM) | Spherical | Gellan gum/GNPs | DOX | Increased cytotoxicity | [25] | |
| 13 ± 5 (TEM) | Spherical | Porphyran/GNPs | DOX | Increased cytotoxicity and targeted release | [30] | |
| 2–4 (TEM) | Spherical | Gum kondagogu/PtNPs | 5-FU | Increased cytotoxicity and sustained release | [23] | |
| Variable sizes depending on incubation time and shape | Spherical/hexagonal/triangular | E. alba aqueous extract/GNPs | DOX | Increased cytotoxicity | [50] | |
| 70.90 ± 8.42 (TEM) | Quasi spherical | Aqueous peel extract of pomegranate/GNPs/FA | 5-FU | Increased cytotoxicity | [54] | |
| Variable sizes depending on the type of MNPs and their shapes | Spherical/hexagonal/triangular | leaf aqueous extract of B. monosperma/GNPs and leaf aqueous extract of B. monosperma/AgNPs | DOX | Increased cytotoxicity | [51] | |
| 54.2 ± 2.1 (DLS) | Spherical | Aqueuous leaf extract of P. pterocarpum/GNPs | DOX | Increased cytotoxicity | [52] | |
| 5–15 (TEM) | Spherical | Almond seed water extract/GNPs/PG9 | Quercetin | Increased cytotoxicity | [56] | |
| 55 ± 3 (DLS) | Spherical | eggplant fruit extract/GNPs/HA | Metformin | Increased cytotoxicity | [53] | |
| 32.7 ± 5.7 (TEM) | Spherical | G. mangostana fruit peel extract/AgNPs | Protocatechuic acid | Increased cytotoxicity | [57] | |
| 20.9 ± 4.4 (TEM) | Spherical | Resveratrol/GNPs | DOX | Increased cytotoxicity | [71] | |
| 11.35 X-ray diffraction | Spherical | Tea ethanolic extract/ZnONPs/Chitosan | Paclitaxel | Targeted release | [59] | |
| 11.3 (TEM) | Spherical | Delftia sp. culture broth/GNPs | Resveratrol | Increased cytotoxicity and targeted release | [88] | |
| 5–20 (TEM) | Spherical | E.coli expressing HMBPs/GNPs | DOX | Targeted release | [87] | |
| 11 ± 5 (TEM) | Spherical | Pullulan/GNPs | Cassiarin A chloride derivatives | Increased cytotoxicity | [39] | |
| 13.7 ± 1.9 (TEM) | Spherical | para-aminobenzoic acid-quat188-pullulan/GNPs | DOX | Increased cytotoxicity | [40] | |
| 12.6 ± 1.5 (TEM) | Spherical | FA-para-aminobenzoic acid-quat188-pullulan/GNPs | DOX | Increased cytotoxicity | [41] | |
| Nanoclusters | 295.2 ± 7.7 (TEM) | Cluster | Green tea/GNCs | DOX | Increased cytotoxicity and targeted and controlled release | [65] |
| Nanoethosomes | 72.4 ± 4.5 (TEM) | Smooth edges and compact | 0.2% EGCG, 2% soybean phosphatidylcholine, 30% ethanol, 1% Tween-80 and 0.1% sugar esters | Docetaxel | Increased cytotoxicity | [81] |
| Quantum dots | 4.9 ± 0.7 (TEM) | Spherical | CMC/(Cu-In-S/ZnS) quantum dots | DOX | Increased cytotoxicity and targeted and controlled release | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbagory, A.M.; Marima, R.M.; Dlamini, Z. Role and Merits of Green Based Nanocarriers in Cancer Treatment. Cancers 2021, 13, 5686. https://doi.org/10.3390/cancers13225686
Elbagory AM, Marima RM, Dlamini Z. Role and Merits of Green Based Nanocarriers in Cancer Treatment. Cancers. 2021; 13(22):5686. https://doi.org/10.3390/cancers13225686
Chicago/Turabian StyleElbagory, Abdulrahman M., Rahaba Makgotso Marima, and Zodwa Dlamini. 2021. "Role and Merits of Green Based Nanocarriers in Cancer Treatment" Cancers 13, no. 22: 5686. https://doi.org/10.3390/cancers13225686