Lung Cancer Surgery after Treatment with Anti-PD1/PD-L1 Immunotherapy for Non-Small-Cell Lung Cancer: A Case—Cohort Study
Abstract
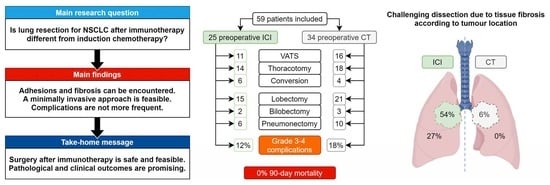
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Tumour Characteristics and Surgical Interventions
3.3. Surgical, Perioperative, and Pathological Outcomes
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol 2018, 36, 1675–1684. [Google Scholar] [CrossRef]
- Noone, A.; Howlader, N.; Krapcho, M. SEER Cancer Statistics Review, 1975–2015; National Cancer Institute: Bethesda, MD, USA, 2018.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; Carpeño, J.D.C.; et al. Neoadjuvant Chemotherapy and Nivolumab in Resectable Non-Small-Cell Lung Cancer (NADIM): An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. ESMO Guidelines Committee Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Hendriks, L.E.L.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Giaj-Levra, M.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of Synchronous Oligometastatic Non–Small Cell Lung Cancer—A Consensus Report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, G.H.M.J.; Toguri, D.; Dahele, M.; Warner, A.; de Haan, P.F.; Rodrigues, G.B.; Slotman, B.J.; Yaremko, B.P.; Senan, S.; Palma, D.A. Radical Treatment of Synchronous Oligometastatic Non-Small Cell Lung Carcinoma (NSCLC): Patient Outcomes and Prognostic Factors. Lung Cancer 2013, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Hellmann, M.D.; Velez, M.J.; Travis, W.D.; Rusch, V.W. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann. Thorac Surg 2017, 104, e217–e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bott, M.J.; Cools-Lartigue, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero Román, A.; Campo-Cañaveral de la Cruz, J.L.; Macía, I.; Escobar Campuzano, I.; Figueroa Almánzar, S.; Delgado Roel, M.; Gálvez Muñoz, C.; García Fontán, E.M.; Muguruza Trueba, I.; Romero Vielva, L.; et al. Outcomes of Surgical Resection after Neoadjuvant Chemoimmunotherapy in Locally Advanced Stage IIIA Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. 2021, 60, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-Related Adverse Events with Immune Checkpoint Blockade: A Comprehensive Review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial Results of Pulmonary Resection after Neoadjuvant Nivolumab in Patients with Resectable Non-Small Cell Lung Cancer. J. Thorac Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Die Loucou, J.; Pagès, P.-B.; Falcoz, P.-E.; Thomas, P.-A.; Rivera, C.; Brouchet, L.; Baste, J.-M.; Puyraveau, M.; Bernard, A.; Dahan, M. Validation and Update of the Thoracic Surgery Scoring System (Thoracoscore) Risk Model. Eur. J. Cardiothorac. Surg. 2020. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Sparrelid, E.; Coppola, A.; Scandavini, C.M.; Joneberg, J.; Segerswärd, R.; Strömberg, C.; Isaksson, B.; Chiaro, M.D. Is There a Real Difference between Grade 3a and 3b in the Clavien–Dindo Classification? A Retrospective Analysis in 1212 Patients Treated at Karolinska University Hospital. HPB 2016, 18, e394–e395. [Google Scholar] [CrossRef] [Green Version]
- Van Schil, P.E.; Rami-Porta, R.; Asamura, H. The 8th TNM Edition for Lung Cancer: A Critical Analysis. Ann. Transl. Med. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Barnett, S.A.; Rusch, V.W.; Zheng, J.; Park, B.J.; Rizk, N.P.; Plourde, G.; Bains, M.S.; Downey, R.J.; Shen, R.; Kris, M.G. Contemporary Results of Surgical Resection of Non-Small Cell Lung Cancer after Induction Therapy: A Review of 549 Consecutive Cases. J. Thorac. Oncol. 2011, 6, 1530–1536. [Google Scholar] [CrossRef] [Green Version]
- Thibout, Y.; Guibert, B.; Bossard, N.; Tronc, F.; Tiffet, O.; de la Roche, E.; Mulsant, P.; Gamondes, J.-P.; Baulieux, J.; Remontet, L.; et al. Is Pneumonectomy After Induction Chemotherapy for Non-Small Cell Lung Cancer a Reasonable Procedure? A Multicenter Retrospective Study of 228 Cases. J. Thorac. Oncol. 2009, 4, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Doddoli, C.; Barlesi, F.; Trousse, D.; Robitail, S.; Yena, S.; Astoul, P.; Giudicelli, R.; Fuentes, P.; Thomas, P. One Hundred Consecutive Pneumonectomies after Induction Therapy for Non-Small Cell Lung Cancer: An Uncertain Balance between Risks and Benefits. J. Thorac. Cardiovasc. Surg. 2005, 130, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schil, P.; Van Meerbeeck, J.; Kramer, G.; Splinter, T.; Legrand, C.; Giaccone, G.; Manegold, C.; van Zandwijk, N. Morbidity and Mortality in the Surgery Arm of EORTC 08941 Trial. Eur. Respir. J. 2005, 26, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boffa, D.; Fernandez, F.G.; Kim, S.; Kosinski, A.; Onaitis, M.W.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Putnam, J.B.; Furnary, A.P. Surgically Managed Clinical Stage IIIA-Clinical N2 Lung Cancer in The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2017, 104, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Goto, T. Role of Surgical Intervention in Unresectable Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 3881. [Google Scholar] [CrossRef] [PubMed]
- Palmero, R.; Vilariño, N.; Navarro-Martín, A.; Nadal, E. Induction Treatment in Patients with Stage III Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 539–554. [Google Scholar] [CrossRef]
- Hoffmann-La Roche. A Phase III, Double-Blinded, Multicenter, Randomized Study Evaluating the Efficacy and Safety of Neoadjuvant Treatment with Atezolizumab or Placebo in Combination with Platinum-Based Chemotherapy in Patients with Resectable Stage II, IIIA, or Select IIIB Non-Small Cell Lung Cancer; clinicaltrials.gov, NCT03456063; Hoffmann-La Roche: Basel, Switzerland, 2021.
- Merck Sharp & Dohme Corp. A Phase III, Randomized, Double-Blind. Trial of Platinum Doublet Chemotherapy +/− Pembrolizumab (MK-3475) as Neoadjuvant/Adjuvant Therapy for Participants with Resectable Stage II, IIIA, and Resectable IIIB (T3-4N2) Non-Small Cell Lung Cancer (NSCLC) (KEYNOTE-671); clinicaltrials.gov; Merck Sharp & Dohme Corp.: Kenilworth, NJ, USA, 2021.
- Bristol-Myers Squibb. Randomized, OpenLabel, Phase 3 Trial of Nivolumab Plus Ipilimumab or Nivolumab Plus Platinum Doublet Chemotherapy Versus Platinum Doublet Chemotherapy in Early Stage NSCLC; clinicaltrials.gov; Bristol-Myers Squibb: New York, NY, USA, 2020.







| Variable | Total Population (n = 59) | ICI Group (n = 25) | Control Group (n = 34) | p |
|---|---|---|---|---|
| Age, years | 60.4 (56–67) | 60.0 (54.6–62.8) | 62.7 (57.6–67.7) | 0.26 |
| BMI, kg/m2 | 24.7 ± 4.6 | 25.2 ± 4.7 | 24.3 ± 4.6 | 0.27 |
| Gender | 0.98 | |||
| Male, n (%) | 40 (67.8) | 17 (68.0) | 23 (67.6) | |
| Female, n (%) | 19 (32.2) | 8 (32.0) | 11 (32.4) | |
| Tobacco status | ||||
| Active or former smoker, n (%) | 55 (93.2) | 23 (92.0) | 32 (94.2) | >0.99 |
| Exposure, pack-years | 38.9 ± 16.0 | 37.8 ± 21.1 | 39.6 ± 11.3 | 0.51 |
| Comorbidities | ||||
| ASA score | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.70 |
| COPD, n (%) | 25 (42.4) | 11 (44.0) | 14 (41.2) | 0.82 |
| Asbestos exposure, n (%) | 9 (15.3) | 3 (12.0) | 6 (17.6) | 0.72 |
| Other respiratory disease *, n (%) | 5 (8.5) | 3 (12.0) | 2 (5.9) | 0.64 |
| Arterial hypertension, n (%) | 22 (37.3) | 10 (40.0) | 12 (35.3) | 0.79 |
| Diabetes, n (%) | 5 (8.5) | 3 (12.0) | 2 (5.9) | 0.64 |
| Dyslipidemia, n (%) | 12 (20.3) | 8 (32.0) | 4 (11.8) | 0.10 |
| History of thoracic surgery, n (%) | 3 (5.1) | 1 (4.0) | 2 (5.9) | >0.99 |
| Pulmonary function testing, n (%) | 59 (100) | 25 (100) | 34 (100) | - |
| FEV1, L | 2.6 ± 0.7 | 2.5 ± 0.6 | 2.6 ± 0.8 | 0.73 |
| FEV1, % predicted | 86.4 ± 16.7 | 85.5 ± 14.8 | 87.2 ± 18.2 | 0.76 |
| FVC, L | 3.6 ± 0.9 | 3.6 ± 0.9 | 3.6 ± 0.9 | 0.78 |
| FVC, % predicted | 99.7 ± 14.5 | 100.0 ± 14.3 | 99.5 ± 14.8 | 0.94 |
| TLC, L | 6.43 ± 1.3 | 6.3 ± 1.4 | 6.6 ± 1.3 | 0.39 |
| TLC, % predicted | 105.2 ± 14.9 | 105.2 ± 14.2 | 105.0 ± 15.7 | 0.89 |
| FEV1/FVC, % | 69.2 ± 9.7 | 69.4 ± 8.7 | 69.1 ± 10.4 | 0.85 |
| TLCO, % predicted | 64.7 ± 15.0 | 64.9 ± 15.8 | 64.6 ± 14.6 | 0.79 |
| Exercise testing, n (%) | 27 (45.8) | 10 (40.0) | 17 (50.0) | 0.60 |
| VO2 max, mL/kg/min | 17.7 ± 3.5 | 17.6 ± 2.1 | 17.8 ± 4.2 | 0.78 |
| Pulmonary rehabilitation, n (%) | 21 (35.6) | 16 (64.0) | 12 (35.3) | 0.03 |
| Histopathology | ||||
| Adenocarcinoma, n (%) | 37 (62.7) | 18 (72.0) | 19 (55.9) | 0.39 |
| Squamous cell carcinoma, n (%) | 20 (33.9) | 6 (24.0) | 14 (41.2) | |
| Carcinoma NOS, n (%) | 2 (33.9) | 1 (4.0) | 1 (2.9) | |
| PD-L1 expression available, n (%) | 40 (67.7) | 20 (80.0) | 20 (58.8) | 0.08 |
| 0% | 13 (22.0) | 3 (12.0) | 10 (29.4) | <0.01 |
| 1–49% | 6 (10.2) | 1 (4.0) | 5 (14.7) | |
| ≥50% | 18 (30.5) | 16 (64.0) | 5 (14.7) |
| Variable | ICI Group (n = 25) | Control Group (n = 34) | p |
|---|---|---|---|
| Location of target lesion | 0.08 | ||
| RUL, n (%) | 7 (28.0) | 15 (44.1) | |
| LUL, n (%) | 11 (44.0) | 6 (17.6) | |
| RLL, n (%) | 2 (8.0) | 8 (23.5) | |
| LLL, n (%) | 5 (20.0) | 5 (14.7) | |
| Topography | 0.38 | ||
| Proximal (juxta-mediastinal), n (%) | 13 (52.0) | 17 (50.0) | |
| Distal, n (%) | 11 (44.0) | 12 (35.2) | |
| Sulcus tumour, n (%) | 1 (4.0) | 5 (14.7) | |
| Size of target lesion on imaging * | |||
| Baseline evaluation, mm | 58.6 ± 26.0 | 63.0 ± 25.9 | |
| Preoperative evaluation, mm | 40.0 ± 21.8 | 44.3 ± 20.9 | |
| Evolution, % | −33.3 (9.0–42.8) | −26.3 (10.7–40.6) | 0.56 |
| Type of resection | 0.41 | ||
| Single lobectomy, n (%) | 15 (60.0) | 21 (61.8) | |
| Pneumonectomy, n (%) | 6 (24.0) | 10 (29.4) | |
| Bilobectomy, n (%) | 2 (8.0) | 3 (8.8) | |
| Segmentectomy, n (%) | 2 (8.0) | 0 | |
| Surgical approach | 0.58 | ||
| VATS only, n (%) | 11 (42.0) | 16 (47.1) | |
| Thoracotomy, n (%) | 14 (58.0) | 18 (52.9) | |
| Robot-assisted surgery, n (%) | 7 (28.0) | 5 (14.7) | |
| Surgery setting | <0.005 | ||
| After neoadjuvant treatment, n (%) | 7 (28.0) | 20 (58.8) | |
| Downstaging, n (%) | 6 (24.0) | 5 (14.7) | |
| Residual tumoral ablation, n (%) | 8 (32.0) | 1 (2.9) | |
| Oligometastatic disease management, n (%) | 4 (16.0) | 8 (23.5) |
| Variable | Preoperative ICI (n = 25) | Control (n = 34) | p |
|---|---|---|---|
| Surgical outcomes | |||
| Operative time, min | 180.6 ± 54.6 | 192.8 ± 68.4 | 0.81 |
| Conversion after VATS, n (%) | 6/17 (35.3) | 4/20 (20.0) | 0.46 |
| Intraoperative mortality, n (%) | 0 | 0 | - |
| Surgeon-reported difficulties | |||
| Challenging dissection, n (%) | 7 (28.0) | 10 (29.4) | 0.91 |
| Adhesions, n (%) | 15 (60.0) | 16 (47.1) | 0.73 |
| Tissue fibrosis or inflammation, n (%) | 10 (40.0) | 1 (2.9) | <0.0004 |
| Intraoperative vascular wound, n (%) | 3 (12.0) | 4 (11.8) | >0.99 |
| Procedure adapted during surgery *, n (%) | 3 (12.0) | 2 (5.9) | 0.64 |
| Perioperative outcomes | |||
| Total hospital LOS, days | 7 (5–11) | 7 (6–9) | 0.74 |
| ICU LOS, days | 1 (0–1] | 1 (0–2) | 0.79 |
| 90-day mortality, n (%) | 0 | 0 | - |
| Perioperative complications—any grade | 12 (48.0) | 16 (47.1) | 0.94 |
| Persistent air leak, n (%) | 4 | 6 | - |
| Pneumonia, n (%) | 6 | 4 | - |
| Recurrent nerve palsy, n (%) | 2 | 5 | - |
| Respiratory failure, n (%) | 2 | 3 | - |
| Persistent pleural effusion, n (%) | 2 | 0 | - |
| Intrathoracic bleeding, n (%) | 1 | 3 | - |
| Arrythmia, n (%) | 2 | 1 | - |
| Atelectasis, n (%) | 0 | 3 | - |
| Sepsis, n (%) | 1 | 1 | - |
| Anastomosis failure, n (%) | 1 | 0 | - |
| Acute urinary retention, n (%) | 0 | 1 | - |
| Pathological outcomes | |||
| R0 achieved, n (%) | 24 (96.0) | 31 (91.2) | 0.63 |
| RVT present, n (%) | 18 (72.0) | 29 (85.2) | 0.33 |
| Major pathological response °, n (%) | 12 (44.0) | 8 (23.5) | 0.05 |
| pCR, n (%) | 7 (28.0) | 5 (14.7) | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Husseini, K.; Piton, N.; De Marchi, M.; Grégoire, A.; Vion, R.; Blavier, P.; Thiberville, L.; Baste, J.-M.; Guisier, F. Lung Cancer Surgery after Treatment with Anti-PD1/PD-L1 Immunotherapy for Non-Small-Cell Lung Cancer: A Case—Cohort Study. Cancers 2021, 13, 4915. https://doi.org/10.3390/cancers13194915
El Husseini K, Piton N, De Marchi M, Grégoire A, Vion R, Blavier P, Thiberville L, Baste J-M, Guisier F. Lung Cancer Surgery after Treatment with Anti-PD1/PD-L1 Immunotherapy for Non-Small-Cell Lung Cancer: A Case—Cohort Study. Cancers. 2021; 13(19):4915. https://doi.org/10.3390/cancers13194915
Chicago/Turabian StyleEl Husseini, Kinan, Nicolas Piton, Marielle De Marchi, Antoine Grégoire, Roman Vion, Pierre Blavier, Luc Thiberville, Jean-Marc Baste, and Florian Guisier. 2021. "Lung Cancer Surgery after Treatment with Anti-PD1/PD-L1 Immunotherapy for Non-Small-Cell Lung Cancer: A Case—Cohort Study" Cancers 13, no. 19: 4915. https://doi.org/10.3390/cancers13194915